Decellularized scaffold of cryopreserved rat kidney retains its recellularization potential
- PMID: 28267813
- PMCID: PMC5340383
- DOI: 10.1371/journal.pone.0173040
Decellularized scaffold of cryopreserved rat kidney retains its recellularization potential
Abstract
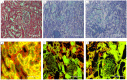
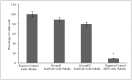
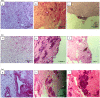
The multi-cellular nature of renal tissue makes it the most challenging organ for regeneration. Therefore, till date whole organ transplantations remain the definitive treatment for the end stage renal disease (ESRD). The shortage of available organs for the transplantation has, thus, remained a major concern as well as an unsolved problem. In this regard generation of whole organ scaffold through decellularization followed by regeneration of the whole organ by recellularization is being viewed as a potential alternative for generating functional tissues. Despite its growing interest, the optimal processing to achieve functional organ still remains unsolved. The biggest challenge remains is the time line for obtaining kidney. Keeping these facts in mind, we have assessed the effects of cryostorage (3 months) on renal tissue architecture and its potential for decellularization and recellularization in comparison to the freshly isolated kidneys. The light microscopy exploiting different microscopic stains as well as immuno-histochemistry and Scanning electron microscopy (SEM) demonstrated that ECM framework is well retained following kidney cryopreservation. The strength of these structures was reinforced by calculating mechanical stress which confirmed the similarity between the freshly isolated and cryopreserved tissue. The recellularization of these bio-scaffolds, with mesenchymal stem cells quickly repopulated the decellularized structures irrespective of the kidneys status, i.e. freshly isolated or the cryopreserved. The growth pattern employing mesenchymal stem cells demonstrated their equivalent recellularization potential. Based on these observations, it may be concluded that cryopreserved kidneys can be exploited as scaffolds for future development of functional organ.
Conflict of interest statement
Figures








References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

