Synthesis of native-like crosslinked duplex RNA and study of its properties
- PMID: 28268052
- PMCID: PMC5969911
- DOI: 10.1016/j.bmc.2017.02.034
Synthesis of native-like crosslinked duplex RNA and study of its properties
Abstract
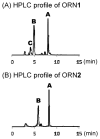
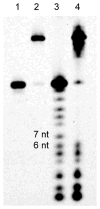
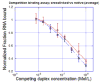
A variety of enzymes have been found to interact with double-stranded RNA (dsRNA) in order to carry out its functions. We have endeavored to prepare the covalently crosslinked native-like duplex RNA, which could be useful for biochemical studies and RNA nanotechnology. In this study, the interstrand covalently linked duplex RNA was formed by a crosslinking reaction between vinylpurine (VP) and the target cytosine or uracil in RNA. We measured melting temperatures and CD spectra to identify the properties of the VP crosslinked duplex RNA. The crosslinking formation increased the thermodynamic stability without disturbing the natural conformation of dsRNA. In addition, a competitive binding experiment with the duplex RNA binding enzyme, ADAR2, showed the crosslinked dsRNA bound the protein with nearly the same binding affinity as the natural dsRNA, confirming that it has finely preserved the natural traits of duplex RNA.
Keywords: 6-Vinylpurine; ADAR; Crosslinking reaction; Native-like; RNA.
Copyright © 2017. Published by Elsevier Ltd.
Figures
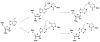





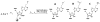
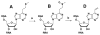
Similar articles
-
Aminoglycoside binding in the major groove of duplex RNA: the thermodynamic and electrostatic forces that govern recognition.J Mol Biol. 2000 Apr 21;298(1):95-110. doi: 10.1006/jmbi.2000.3639. J Mol Biol. 2000. PMID: 10756107
-
Incorporating uracil and 5-halouracils into short peptide nucleic acids for enhanced recognition of A-U pairs in dsRNAs.Nucleic Acids Res. 2018 Sep 6;46(15):7506-7521. doi: 10.1093/nar/gky631. Nucleic Acids Res. 2018. PMID: 30011039 Free PMC article.
-
A Short Chemically Modified dsRNA-Binding PNA (dbPNA) Inhibits Influenza Viral Replication by Targeting Viral RNA Panhandle Structure.Bioconjug Chem. 2019 Mar 20;30(3):931-943. doi: 10.1021/acs.bioconjchem.9b00039. Epub 2019 Feb 15. Bioconjug Chem. 2019. PMID: 30721034
-
Recognition of double-stranded RNA by proteins and small molecules.Biopolymers. 2003 Sep;70(1):86-102. doi: 10.1002/bip.10413. Biopolymers. 2003. PMID: 12925995 Review.
-
The NFAR's (nuclear factors associated with dsRNA): evolutionarily conserved members of the dsRNA binding protein family.RNA Biol. 2009 Jan-Mar;6(1):35-9. doi: 10.4161/rna.6.1.7565. Epub 2009 Jan 3. RNA Biol. 2009. PMID: 19106622 Review.
Cited by
-
Function Control of Anti-microRNA Oligonucleotides Using Interstrand Cross-Linked Duplexes.Mol Ther Nucleic Acids. 2018 Mar 2;10:64-74. doi: 10.1016/j.omtn.2017.11.003. Epub 2017 Nov 14. Mol Ther Nucleic Acids. 2018. PMID: 29499957 Free PMC article.
References
-
- Beal PA. ChemBioChem. 2005;6:257. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

