Nanoporous membrane robustness / stability in small form factor microfluidic filtration system
- PMID: 28268711
- PMCID: PMC6390479
- DOI: 10.1109/EMBC.2016.7591106
Nanoporous membrane robustness / stability in small form factor microfluidic filtration system
Abstract
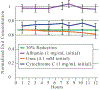
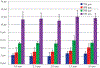
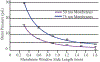
The development of wearable hemodialysis (HD) devices that replace center-based HD holds the promise to improve both outcomes and quality-of-life for patients with end-stage-renal disease (ERD). A prerequisite for these devices is the development of highly efficient membranes that can achieve high toxin clearance in small footprints. The ultrathin nanoporous membrane material developed by our group is orders of magnitude more permeable than conventional HD membranes. We report on our progress making a prototype wearable dialysis unit. First, we present data from benchtop studies confirming that clinical levels of urea clearance can be obtained in a small animal model with low blood flow rates. Second, we report on efforts to improve the mechanical robustness of high membrane area dialysis devices.
Figures



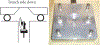
References
-
- Blagg CR, “The 50th anniversary of long-term hemodialysis: University of Washington Hospital, March 9th, 1960,” in J Nephrol vol. 24 Suppl 17, ed Italy, 2011, pp. S84–8. - PubMed
-
- Striemer CC, Gaborski TR, McGrath JL, and Fauchet PM, “Charge- and size-based separation of macromolecules using ultrathin silicon membranes,” Nature, vol. 445, pp. 749–53, February 2007. - PubMed
-
- Agrawal AA, Nehilla BJ, Reisig KV, Gaborski TR, Fang DZ, Striemer CC, et al., “Porous nanocrystalline silicon membranes as highly permeable and molecularly thin substrates for cell culture,” Biomaterials, vol. 31, pp. 5408–17, July 2010. - PubMed
-
- Getpreecharsawas J, McGrath JL, and Borkholder DA, “The electric field strength in orifice-like nanopores of ultrathin membranes,” Nanotechnology, vol. 26, p. 045704, January 2015. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
