Automatic data source identification for clinical trial eligibility criteria resolution
- PMID: 28269912
- PMCID: PMC5333255
Automatic data source identification for clinical trial eligibility criteria resolution
Abstract
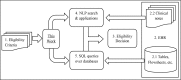
Clinical trial coordinators refer to both structured and unstructured sources of data when evaluating a subject for eligibility. While some eligibility criteria can be resolved using structured data, some require manual review of clinical notes. An important step in automating the trial screening process is to be able to identify the right data source for resolving each criterion. In this work, we discuss the creation of an eligibility criteria dataset for clinical trials for patients with two disparate diseases, annotated with the preferred data source for each criterion (i.e., structured or unstructured) by annotators with medical training. The dataset includes 50 heart-failure trials with a total of 766 eligibility criteria and 50 trials for chronic lymphocytic leukemia (CLL) with 677 criteria. Further, we developed machine learning models to predict the preferred data source: kernel methods outperform simpler learning models when used with a combination of lexical, syntactic, semantic, and surface features. Evaluation of these models indicates that the performance is consistent across data from both diagnoses, indicating generalizability of our method. Our findings are an important step towards ongoing efforts for automation of clinical trial screening.
Figures
Similar articles
-
Textual inference for eligibility criteria resolution in clinical trials.J Biomed Inform. 2015 Dec;58 Suppl(Suppl):S211-S218. doi: 10.1016/j.jbi.2015.09.008. Epub 2015 Sep 14. J Biomed Inform. 2015. PMID: 26376462 Free PMC article.
-
Increasing the efficiency of trial-patient matching: automated clinical trial eligibility pre-screening for pediatric oncology patients.BMC Med Inform Decis Mak. 2015 Apr 14;15:28. doi: 10.1186/s12911-015-0149-3. BMC Med Inform Decis Mak. 2015. PMID: 25881112 Free PMC article.
-
Automated classification of eligibility criteria in clinical trials to facilitate patient-trial matching for specific patient populations.J Am Med Inform Assoc. 2017 Jul 1;24(4):781-787. doi: 10.1093/jamia/ocw176. J Am Med Inform Assoc. 2017. PMID: 28339690 Free PMC article.
-
A review of research on eligibility criteria for clinical trials.Clin Exp Med. 2023 Oct;23(6):1867-1879. doi: 10.1007/s10238-022-00975-1. Epub 2023 Jan 5. Clin Exp Med. 2023. PMID: 36602707 Free PMC article. Review.
-
Machine learning and natural language processing in clinical trial eligibility criteria parsing: a scoping review.Drug Discov Today. 2024 Oct;29(10):104139. doi: 10.1016/j.drudis.2024.104139. Epub 2024 Aug 19. Drug Discov Today. 2024. PMID: 39154773
Cited by
-
Artificial Intelligence Applied to clinical trials: opportunities and challenges.Health Technol (Berl). 2023;13(2):203-213. doi: 10.1007/s12553-023-00738-2. Epub 2023 Feb 28. Health Technol (Berl). 2023. PMID: 36923325 Free PMC article. Review.
-
Sociotechnical feasibility of natural language processing-driven tools in clinical trial eligibility prescreening for Alzheimer's disease and related dementias.J Am Med Inform Assoc. 2024 Apr 19;31(5):1062-1073. doi: 10.1093/jamia/ocae032. J Am Med Inform Assoc. 2024. PMID: 38447587 Free PMC article.
-
Digital tools for the recruitment and retention of participants in randomised controlled trials: a systematic map.Trials. 2020 Jun 5;21(1):478. doi: 10.1186/s13063-020-04358-3. Trials. 2020. PMID: 32498690 Free PMC article. Review.
-
Use of Natural Language Processing to Extract Clinical Cancer Phenotypes from Electronic Medical Records.Cancer Res. 2019 Nov 1;79(21):5463-5470. doi: 10.1158/0008-5472.CAN-19-0579. Epub 2019 Aug 8. Cancer Res. 2019. PMID: 31395609 Free PMC article. Review.
-
Automated NLP Extraction of Clinical Rationale for Treatment Discontinuation in Breast Cancer.JCO Clin Cancer Inform. 2021 May;5:550-560. doi: 10.1200/CCI.20.00139. JCO Clin Cancer Inform. 2021. PMID: 33989016 Free PMC article.
References
-
- Prokosch HU, Ganslandt T. Perspectives for medical informatics. Reusing the electronic medical record for clinical research. Methods Inf Med. 2009 Jan.48(1):38–44. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical