Oncometabolites d- and l-2-Hydroxyglutarate Inhibit the AlkB Family DNA Repair Enzymes under Physiological Conditions
- PMID: 28269980
- PMCID: PMC5498157
- DOI: 10.1021/acs.chemrestox.7b00009
Oncometabolites d- and l-2-Hydroxyglutarate Inhibit the AlkB Family DNA Repair Enzymes under Physiological Conditions
Abstract
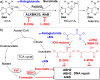
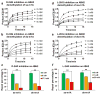
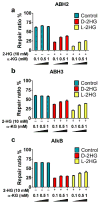
Cancer-associated mutations often lead to perturbed cellular energy metabolism and accumulation of potentially harmful oncometabolites. One example is the chiral molecule 2-hydroxyglutarate (2HG); its two stereoisomers (d- and l-2HG) have been found at abnormally high concentrations in tumors featuring anomalous metabolic pathways. 2HG has been demonstrated to competitively inhibit several α-ketoglutarate (αKG)- and non-heme iron-dependent dioxygenases, including some of the AlkB family DNA repair enzymes, such as ALKBH2 and ALKBH3. However, previous studies have only provided the IC50 values of d-2HG on the enzymes, and the results have not been correlated to physiologically relevant concentrations of 2HG and αKG in cancer cells. In this work, we performed detailed kinetic analyses of DNA repair reactions catalyzed by ALKBH2, ALKBH3, and the bacterial AlkB in the presence of d- and l-2HG in both double- and single-stranded DNA contexts. We determined the kinetic parameters of inhibition, including kcat, KM, and Ki. We also correlated the relative concentrations of 2HG and αKG previously measured in tumor cells with the inhibitory effect of 2HG on the AlkB family enzymes. Both d- and l-2HG significantly inhibited the human DNA repair enzymes ALKBH2 and ALKBH3 at pathologically relevant concentrations (73-88% for d-2HG and 31-58% for l-2HG inhibition). This work provides a new perspective that the elevation of the d- or l-2HG concentration in cancer cells may contribute to an increased mutation rate by inhibiting the DNA repair performed by the AlkB family enzymes and thus exacerbate the genesis and progression of tumors.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Rhein Inhibits AlkB Repair Enzymes and Sensitizes Cells to Methylated DNA Damage.J Biol Chem. 2016 May 20;291(21):11083-93. doi: 10.1074/jbc.M115.711895. Epub 2016 Mar 25. J Biol Chem. 2016. PMID: 27015802 Free PMC article.
-
DNA repair enzymes ALKBH2, ALKBH3, and AlkB oxidize 5-methylcytosine to 5-hydroxymethylcytosine, 5-formylcytosine and 5-carboxylcytosine in vitro.Nucleic Acids Res. 2019 Jun 20;47(11):5522-5529. doi: 10.1093/nar/gkz395. Nucleic Acids Res. 2019. PMID: 31114894 Free PMC article.
-
"Repair and fold" DNA nanotweezers for measuring DNA alkylation repair mediated by ALKBH.Analyst. 2025 Mar 24;150(7):1229-1234. doi: 10.1039/d5an00099h. Analyst. 2025. PMID: 40034040
-
Roles of the oncometabolite enantiomers of 2-hydroxyglutarate and their metabolism by diverse dehydrogenases.Essays Biochem. 2024 Oct 3;68(2):161-171. doi: 10.1042/EBC20230077. Essays Biochem. 2024. PMID: 38919140 Review.
-
Mitochondrial 2-hydroxyglutarate metabolism.Mitochondrion. 2014 Nov;19 Pt B:275-81. doi: 10.1016/j.mito.2014.02.009. Epub 2014 Feb 21. Mitochondrion. 2014. PMID: 24561575 Review.
Cited by
-
An Ultrasensitive Biosensor for Probing Subcellular Distribution and Mitochondrial Transport of l-2-Hydroxyglutarate.Adv Sci (Weinh). 2024 Sep;11(35):e2404119. doi: 10.1002/advs.202404119. Epub 2024 Jul 15. Adv Sci (Weinh). 2024. PMID: 39005231 Free PMC article.
-
Beyond Brooding on Oncometabolic Havoc in IDH-Mutant Gliomas and AML: Current and Future Therapeutic Strategies.Cancers (Basel). 2018 Feb 11;10(2):49. doi: 10.3390/cancers10020049. Cancers (Basel). 2018. PMID: 29439493 Free PMC article. Review.
-
Kinetic Studies on the 2-Oxoglutarate/Fe(II)-Dependent Nucleic Acid Modifying Enzymes from the AlkB and TET Families.DNA (Basel). 2023 Jun;3(2):65-84. doi: 10.3390/dna3020005. Epub 2023 Mar 30. DNA (Basel). 2023. PMID: 38698914 Free PMC article.
-
Magnetic resonance spectroscopy for the study of cns malignancies.Prog Nucl Magn Reson Spectrosc. 2021 Feb;122:23-41. doi: 10.1016/j.pnmrs.2020.11.001. Epub 2020 Dec 2. Prog Nucl Magn Reson Spectrosc. 2021. PMID: 33632416 Free PMC article.
-
Control of endothelial quiescence by FOXO-regulated metabolites.Nat Cell Biol. 2021 Apr;23(4):413-423. doi: 10.1038/s41556-021-00637-6. Epub 2021 Apr 1. Nat Cell Biol. 2021. PMID: 33795871 Free PMC article.
References
-
- Parsons DW, Jones S, Zhang X, Lin JC-H, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu I-M, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Hartigan J, Smith DR, Strausberg RL, Marie SKN, Shinjo SMO, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. - PMC - PubMed
-
- Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, Koboldt DC, Fulton RS, Delehaunty KD, McGrath SD, Fulton LA, Locke DP, Magrini VJ, Abbott RM, Vickery TL, Reed JS, Robinson JS, Wylie T, Smith SM, Carmichael L, Eldred JM, Harris CC, Walker J, Peck JB, Du F, Dukes AF, Sanderson GE, Brummett AM, Clark E, McMichael JF, Meyer RJ, Schindler JK, Pohl CS, Wallis JW, Shi X, Lin L, Schmidt H, Tang Y, Haipek C, Wiechert ME, Ivy JV, Kalicki J, Elliott G, Ries RE, Payton JE, Westervelt P, Tomasson MH, Watson MA, Baty J, Heath S, Shannon WD, Nagarajan R, Link DC, Walter MJ, Graubert TA, DiPersio JF, Wilson RK, Ley TJ. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 2009;361:1058–1066. - PMC - PubMed
-
- Cairns RA, Mak TW. Oncogenic isocitrate dehydrogenase mutations: mechanisms, models, and clinical opportunities. Cancer Discov. 2013;3:730–741. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

