Molecules in pain and sex: a developing story
- PMID: 28270169
- PMCID: PMC5341415
- DOI: 10.1186/s13041-017-0289-8
Molecules in pain and sex: a developing story
Abstract
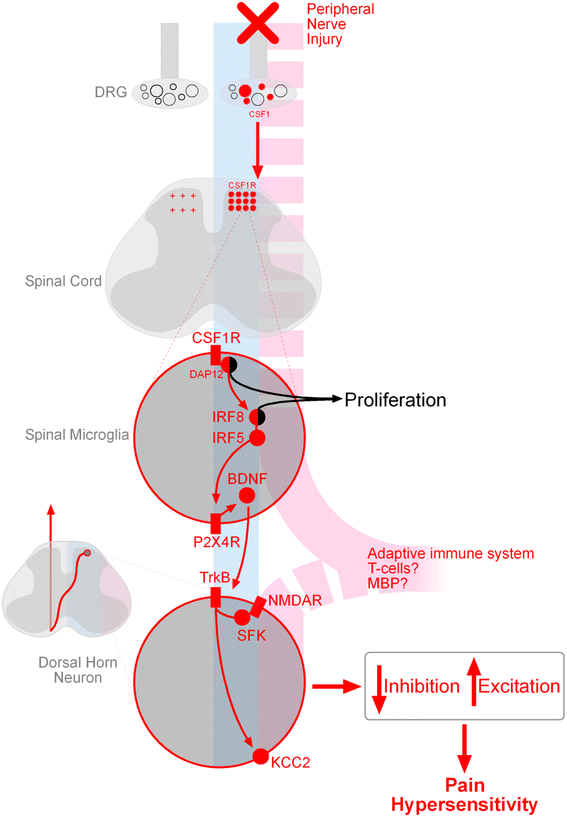
Microglia are dynamic immune cells with diverse roles in maintaining homeostasis of the central nervous system. Dysregulation of microglia has been critically implicated in the genesis of neuropathic pain. Peripheral nerve injury, a common cause of neuropathic pain, engages microglia-neuronal signalling which causes disinhibition and facilitated excitation of spinal nociceptive pathways. However, recent literature indicates that the role of microglia in neuropathic pain is sexually dimorphic, and that female pain processing appears to be independent of microglia, depending rather on T cells. Despite this sex difference, pain signalling in the spinal cord converges downstream of microglia, as NMDAR-mediated facilitated excitation in pain transmitting neurons is consistent between males and females. Determining whether pain signalling is sexually dimorphic in humans and, further, addressing the sex bias in pain research will increase the translational relevance of preclinical findings and advance our understanding of chronic pain in women.
Keywords: Microglia; Neuropathic Pain; Sex differences; Spinal Cord; T cells.
Figures
References
-
- Pizzo P, Clark N. Relieving pain in America: a blueprint for transforming prevention, care, education, and research (Institute of medicine) Washington, DC: The National Academies Press; 2011. - PubMed
-
- Merskey H, Bogduk N. Classification of chronic pain. 2. Seattle: IASP Press; 1994.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

