Genetic and epigenetic stability of oligodendrogliomas at recurrence
- PMID: 28270234
- PMCID: PMC5339990
- DOI: 10.1186/s40478-017-0422-z
Genetic and epigenetic stability of oligodendrogliomas at recurrence
Abstract
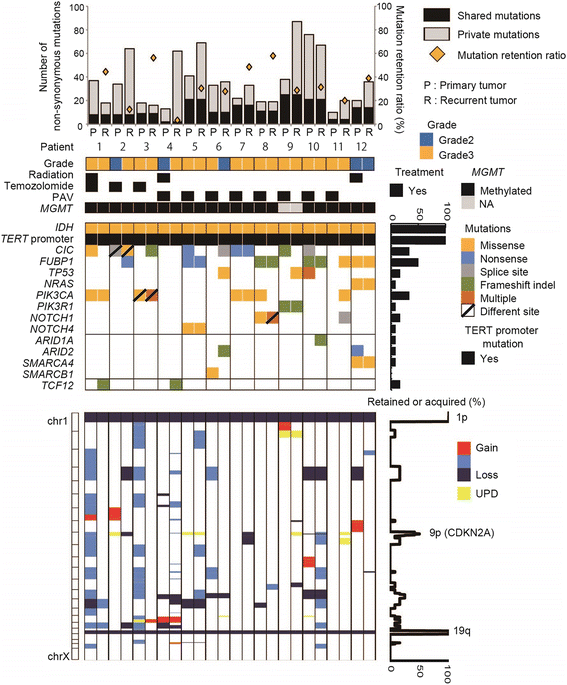
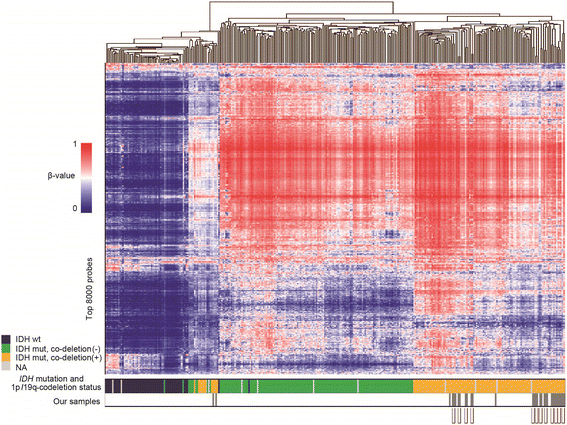
Among diffuse gliomas, oligodendrogliomas show relatively better prognosis, respond well to radiotherapy and chemotherapy, and seldom progress to very aggressive tumors. To elucidate the genetic and epigenetic background for such behavior and tumor evolution during tumor relapse, we comparatively analyzed 12 pairs of primary and recurrent oligodendrogliomas with 1p/19q-codeletion. Initial treatment for these patients was mostly chemotherapy alone. Temozolomide was used for 3, and procarbazine, nimustine and vincristine (PAV chemotherapy) were used for 7 patients. World Health Organization histological grade at recurrence was mostly stable; it was increased in 2, the same in 9, and decreased in 1 cases. Whole-exome sequencing demonstrated that the rate of shared mutation between the primary and recurrent tumors was relatively low, ranging from 3.2-57.9% (average, 33.3%), indicating a branched evolutionary pattern. The trunk alterations that existed throughout the course were restricted to IDH1 mutation, 1p/19q-codeletion, and TERT promoter mutation, and mutation of the known candidate tumor suppressor genes CIC and FUBP1 were not consistently observed between primary and recurrent tumors. Multiple sampling from different regions within a tumor showed marked intratumoral heterogeneity. Notably, in general, the number of mutations was not significantly different after recurrence, remaining under 100, and no hypermutator phenotype was observed. FUBP1 mutation, loss of chr. 9p21, and TCF12 mutation were among a few recurrent de novo alterations that were found at recurrence, indicating that these events were clonally selected at recurrence but were not enough to enhance malignancy. Genome-wide methylation status, measured by Illumina 450 K arrays, was stable between recurrence and the primary tumor. In summary, although oligodendroglioma displays marked mutational heterogeneity, histological malignant transformation accompanying events such as considerable increase in mutation number and epigenetic profile change were not observed at recurrence, indicating that noticeable temporal and spatial genetic heterogeneity in oligodendrogliomas does not result in rapid tumor progression.
Keywords: Heterogeneity; Hypermutator; Methylation; Mutation; Oligodendroglioma.
Figures


References
-
- Alentorn A, Dehais C, Ducray F, Carpentier C, Mokhtari K, Figarella-Branger D, Chinot O, Cohen-Moyal E, Ramirez C, Loiseau H, et al. Allelic loss of 9p21.3 is a prognostic factor in 1p/19q codeleted anaplastic gliomas. Neurology. 2015;85:1325–1331. doi: 10.1212/WNL.0000000000002014. - DOI - PMC - PubMed
-
- Bady P, Sciuscio D, Diserens AC, Bloch J, van den Bent MJ, Marosi C, Dietrich PY, Weller M, Mariani L, Heppner FL, et al. MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta Neuropathol. 2012;124:547–560. doi: 10.1007/s00401-012-1016-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

