Protein ligands for studying ion channel proteins
- PMID: 28270405
- PMCID: PMC5379924
- DOI: 10.1085/jgp.201711776
Protein ligands for studying ion channel proteins
Abstract
Chavan et al. highlight work showing that a monobody can inhibit a fluoride channel using a mechanism similar to that of a scorpion toxin blocker of potassium channels.
Figures
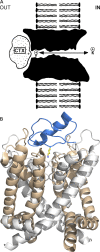

Comment on
-
Mechanism of single- and double-sided inhibition of dual topology fluoride channels by synthetic monobodies.J Gen Physiol. 2017 Apr 3;149(4):511-522. doi: 10.1085/jgp.201611747. Epub 2017 Mar 3. J Gen Physiol. 2017. PMID: 28258203 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

