Essential role of the flexible linker on the conformational equilibrium of bacterial peroxiredoxin reductase for effective regeneration of peroxiredoxin
- PMID: 28270505
- PMCID: PMC5399115
- DOI: 10.1074/jbc.M117.775858
Essential role of the flexible linker on the conformational equilibrium of bacterial peroxiredoxin reductase for effective regeneration of peroxiredoxin
Abstract
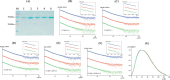
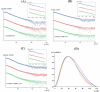
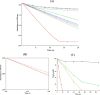
Reactive oxygen species (ROS) can damage DNA, proteins, and lipids, so cells have antioxidant systems that regulate ROS. In many bacteria, a dedicated peroxiredoxin reductase, alkyl hydroperoxide reductase subunit F (AhpF), catalyzes the rapid reduction of the redox-active disulfide center of the antioxidant protein peroxiredoxin (AhpC) to detoxify ROS such as hydrogen peroxide, organic hydroperoxide, and peroxynitrite. AhpF is a flexible multidomain protein that enables a series of electron transfers among the redox centers by accepting reducing equivalents from NADH. A flexible linker connecting the N-terminal domain (NTD) and C-terminal domain (CTD) of AhpF suggests that the enzyme adopts a large-scale domain motion that alternates between the closed and open states to shuttle electrons from the CTD via the NTD to AhpC. Here, we conducted comprehensive mutational, biochemical, and biophysical analyses to gain insights into the role of the flexible linker and the residues critical for the domain motions of Escherichia coli AhpF (EcAhpF) during electron transfer. Small-angle X-ray scattering studies of linker mutants revealed that a group of charged residues, 200EKR202, is crucial for the swiveling motion of the NTD. Moreover, NADH binding significantly affected EcAhpF flexibility and the movement of the NTD relative to the CTD. The mutants also exhibited a decrease in H2O2 reduction by the AhpF-AhpC ensemble. We propose that a concerted movement involving the NTD, C-terminal NADH, and FAD domains, and the flexible linker between them is essential for optimal intra-domain cross-talk and for efficient electron transfer to the redox partner AhpC required for peroxidation.
Keywords: antioxidant; bioenergetics; electron transfer; enzyme mechanism; flavoprotein; protein structure; small-angle X-ray scattering (SAXS).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
-
- D'Autréaux B., and Toledano M. B. (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824 - PubMed
-
- Winterbourn C. C. (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat. Chem. Biol. 4, 278–286 - PubMed
-
- Jacobson F. S., Morgan R. W., Christman M. F., and Ames B. N. (1989) An alkyl hydroperoxide reductase from Salmonella typhimurium involved in the defense of DNA against oxidative damage: purification and properties. J. Biol. Chem. 264, 1488–1496 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

