Structure and specificity of a new class of Ca2+-independent housekeeping sortase from Streptomyces avermitilis provide insights into its non-canonical substrate preference
- PMID: 28270507
- PMCID: PMC5409490
- DOI: 10.1074/jbc.M117.782037
Structure and specificity of a new class of Ca2+-independent housekeeping sortase from Streptomyces avermitilis provide insights into its non-canonical substrate preference
Abstract
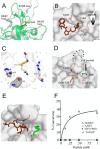
Surface proteins in Gram-positive bacteria are incorporated into the cell wall through a peptide ligation reaction catalyzed by transpeptidase sortase. Six main classes (A-F) of sortase have been identified of which class A sortase is meant for housekeeping functions. The prototypic housekeeping sortase A (SaSrtA) from Staphylococcus aureus cleaves LPXTG-containing proteins at the scissile T-G peptide bond and ligates protein-LPXT to the terminal Gly residue of the nascent cross-bridge of peptidoglycan lipid II precursor. Sortase-mediated ligation ("sortagging") of LPXTG-containing substrates and Gly-terminated nucleophiles occurs in vitro as well as in cellulo in the presence of Ca2+ and has been applied extensively for protein conjugations. Although the majority of applications emanate from SaSrtA, low catalytic efficiency, LPXTG specificity restriction, and Ca2+ requirement (particularly for in cellulo applications) remain a drawback. Given that Gram-positive bacteria genomes encode a variety of sortases, natural sortase mining can be a viable complementary approach akin to engineering of wild-type SaSrtA. Here, we describe the structure and specificity of a new class E sortase (SavSrtE) annotated to perform housekeeping roles in Streptomyces avermitilis Biochemical experiments define the attributes of an optimum peptide substrate, demonstrate Ca2+-independent activity, and provide insights about contrasting functional characteristics of SavSrtE and SaSrtA. Crystal structure, substrate docking, and mutagenesis experiments have identified a critical residue that dictates the preference for a non-canonical LAXTG recognition motif over LPXTG. These results have implications for rational tailoring of substrate tolerance in sortases. Besides, Ca2+-independent orthogonal specificity of SavSrtE is likely to expand the sortagging toolkit.
Keywords: Streptomyces; crystal structure; enzyme catalysis; peptide; peptides; sortase A; substrate specificity; transpeptidase.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
-
- Mazmanian S. K., Ton-That H., and Schneewind O. (2001) Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol. Microbiol. 40, 1049–1057 - PubMed
-
- Mazmanian S. K., Liu G., Ton-That H., and Schneewind O. (1999) Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 285, 760–763 - PubMed
-
- Novick R. P. (2000) Sortase: the surface protein anchoring transpeptidase and the LPXTG motif. Trends Microbiol. 8, 148–151 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

