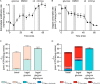
Figure 1.
Maximum extramitochondrial ATP yields and P/O ratios for the catabolism of conventional substrates by isolated mammalian mitochondria and physiological substrates by mammalian cells and calculation of the rates of ATP production from glycolysis, tricarboxylic acid cycle, β-oxidation, and oxidative phosphorylation using oxygen consumption rate.
A, maximum extramitochondrial ATP yields and P/O ratios. The maximum net yield of glycolytic ATP/mol of glucose or glycogen converted to pyruvate (and then to lactate or oxidized through the TCA cycle), ATPglyc, is given in column g, after correction for ATP used to activate glucose or glycogen (calculated using columns b and c and columns e and f). Column o gives ATPox, the maximum oxidative yield of ATP/mol of substrate oxidized by pyruvate dehydrogenase, TCA cycle, β-oxidation, and electron transport chain, including substrate-linked phosphorylation in the TCA cycle and NADH equivalents imported from glycolytic reactions, and corrected for ATP used to activate substrates other than glucose (calculated using columns b and c and columns h and n). Values are given with bars (i.e. 1.63 = 1.636363 …) to emphasize that they are not integers or approximations but exact values arising from the arithmetic of small integers as shown (values for glycogen incorporate an assumption about branching, so they are less precise and are therefore rounded to two decimal places). Column p gives the maximum total yield of ATP per mol substrate (sum of columns g and o). Column q expresses this maximum yield of ATP per mol of oxygen atoms [O] consumed (i.e. the maximum P/O ratio for the reactions in column b). The values in each column are calculated row-by-row as follows. During oxidation of succinate to malate by isolated mitochondria, succinate enters on the dicarboxylate carrier in exchange for malate and is oxidized to fumarate by succinate dehydrogenase, reducing Q to QH2. QH2 is oxidized by the electron transport chain, reducing 1 [O] to H2O and driving 6 H+ from the matrix to the intermembrane space. The fumarate is hydrated to malate by fumarase and exits in exchange for incoming succinate. The 6 H+ re-enter the matrix through the ATP synthesis machinery, which translocates 8 H+ through the ATP synthase and 3 H+ through the phosphate and adenine nucleotide carriers for every 3 ATP generated, giving an H+/ATP ratio of 11:3 (see “Results”). In this way, the oxidation of succinate causes phosphorylation of 6 H+/O × 3/11 ATP/H+ = 1.63 ATP molecules/[O] reduced to water. This is the maximum P/O ratio for oxidation of succinate by mitochondria. During oxidation of glycerol 3-phosphate to dihydroxyacetone phosphate by isolated mitochondria, mitochondrial glycerol 3-phosphate dehydrogenase reduces Q to QH2, which is oxidized as above, with the same P/Omax of 1.63. During oxidation of pyruvate plus malate by isolated mitochondria, pyruvate enters mitochondria on the pyruvate carrier (electroneutrally with a proton) and is oxidized to acetyl-CoA and CO2 by pyruvate dehydrogenase. Malate enters in exchange for citrate and is oxidized to oxaloacetate by malate dehydrogenase. The two dehydrogenation reactions form a total of 2 NADH. Citrate synthase uses acetyl-CoA and oxaloacetate to form citrate, which exits the mitochondria with a proton (balancing the proton imported with pyruvate) on the tricarboxylate carrier in exchange for incoming malate. The 2 NADH are oxidized, driving pumping of 20 H+ and generating 20 × 3/11 = 5.45 ATP with a P/Omax of 2.72. During oxidation of malate plus glutamate by isolated mitochondria, malate enters electroneutrally on the dicarboxylate carrier in exchange for 2-oxoglutarate, and glutamate enters on the glutamate-aspartate carrier electrogenically with a proton (which is therefore unavailable for ATP synthesis; column l) in exchange for aspartate. Malate dehydrogenase produces oxaloacetate plus 1 NADH, which is oxidized, driving pumping of 10 H+, and then aspartate aminotransferase uses oxaloacetate and glutamate to generate 2-oxoglutarate and aspartate, which exit in exchange for incoming malate and glutamate. Overall, 9 H+ are translocated, driving synthesis of 9 × 3/11 = 2.45 ATP/[O]. During glycolysis of glucose to lactate by cells, 1 ATP is consumed at hexokinase, and 1 ATP is consumed at phosphofructokinase to yield 2 trioses, each of which generates 1 ATP at phosphoglycerate kinase and 1 ATP at pyruvate kinase, for a net yield of 2 ATP/glucose. The 2 NADH generated at glyceraldehyde phosphate dehydrogenase are reoxidized during reduction of pyruvate to lactate by lactate dehydrogenase, and 2 lactates are exported from the cell accompanied by the 2 H+ generated by the conversion of 1 uncharged glucose to 2 anionic lactate− molecules. Overall, glycolysis to lactate produces 2 ATP/glucose (1 ATP/lactate). During glycolysis of glucose to pyruvate by cells followed by oxidation of pyruvate to bicarbonate by pyruvate dehydrogenase and the TCA cycle, 2 ATP are formed by glycolytic reactions per glucose. In addition, 2 ATP are formed in the mitochondrial matrix during oxidative metabolism by the substrate-linked reaction at succinyl-CoA synthetase. Each of these ATPs is exported to the cytosol, using 1 H+ in the process, giving a net cytosolic ATP yield from succinyl-CoA synthetase of 2 × (1 − 3/11) = 1.45 ATP/glucose. NADH generated at glyceraldehyde phosphate dehydrogenase enters the mitochondria on the malate-aspartate shuttle, driven by re-entry of 2 of the 20 subsequently translocated H+ (column l), or on the glycerol 3-phosphate shuttle (which allows the reducing equivalents to enter the electron transport chain without passing through complex I, so pumping 12 H+, 8 fewer than normal for matrix NADH; column l). The 2 pyruvates from glycolysis are fully oxidized by pyruvate dehydrogenase and the TCA cycle, generating 8 NADH and 2 QH2 (driving pumping of 92 H+). The sum of 110 (or 104 if the glycerol 3-phosphate shuttle is used) translocated H+ yields a maximum of 110 × 3/11 = 30 (or 104 × 3/11 = 28.36 ATP, which, together with the substrate-linked ATP production, gives a net oxidative yield of 31.45 (or 28.81) ATP/glucose. The overall ATP yield is the sum of the glycolytic and oxidative yields: 33.45 (or 31.81) ATP/glucose or a P/Omax of 2.78 (or 2.651). During catabolism of glycogen, the yields are the same as those for catabolism of glucose, except that less ATP is needed for the initial activation reactions. About 90% of the linkages in glycogen are α-1,4, which are split by the addition of phosphate, yielding glucose 1-phosphate and bypassing the consumption of ATP at hexokinase. The remainder are α-1,6, which are hydrolyzed to yield glucose, which requires activation at hexokinase. On average, activation therefore requires ∼0.1 ATP at hexokinase and 1 ATP at phosphofructokinase, increasing the ATP yield of glycogen catabolism by ∼0.9 ATP/glucose compared with catabolism of glucose itself, giving the yields of ATP/glucose unit and P/Omax ratios shown. Complete oxidation of pyruvate by cells bypasses glycolysis and generates 1 ATP/pyruvate at succinyl-CoA synthetase in the matrix (0.72 ATP/pyruvate after export of ATP). Proton pumping yields 46 × 3/11 = 12.54 ATP/pyruvate, for a sum of 13.27 ATP/pyruvate or a P/Omax of 2.654. During complete oxidation of palmitate by cells, palmitate activation to palmitoyl-CoA generates AMP from ATP, effectively using 2 ATP/palmitate. Palmitoyl-CoA enters the matrix electroneutrally as palmitoyl carnitine on the carnitine transporter, and then β-oxidation to 8 acetyl-CoA yields 7 NADH and 7 QH2, and oxidation of 8 acetyl-CoA in the TCA cycle yields 24 NADH, 8 QH2, and 8 matrix ATP. The maximum overall yield is 112.90 ATP/palmitate and a P/Omax of 2.45. Oxidation of other fatty acids gives slightly different yields and P/Omax values; the monounsaturated oleate, whose oxidation generates 1 fewer QH2 and 6 fewer H+ translocated than the corresponding saturated fatty acid, is calculated out as an example. HK, hexokinase; PFK, phosphofructokinase; PK, pyruvate kinase; PGK, phosphoglycerate kinase; ACS, acyl- CoA synthase; SCS, succinyl-CoA synthetase. Purple highlights isolated mitochondria; gray highlights cells (and the darker band highlights complete oxidation of glucose by cells). B, calculation of yields of ATP per oxygen consumed. Non-repeating values are rounded to two decimal places for assumed estimates and to three decimal places for real numbers. Columns s–u divide the overall P/O ratio in A (column q) into components dependent on different subsets of the total mitochondrial oxygen consumption, to enable calculations of glycolytic and oxidative ATP yields from experimental oxygen consumption data (glycolytic ATP yields from glycolysis to lactate are calculated from extracellular acidification, but glycolytic ATP yields from glycolysis to pyruvate (subsequently oxidized) are calculated from oxygen consumption). Column s gives the glycolytic ATP yield from conversion of glucose or glycogen to pyruvate subsequently oxidized to bicarbonate (which depends on total mitochondrial oxygen consumption). Column t gives the P/O ratio for substrate-linked reactions in the TCA cycle and β-oxidation (which depends on total mitochondrial oxygen consumption), and column u gives the P/O ratio for oxidation of NADH and QH2 derived from pyruvate dehydrogenase, TCA cycle, and β-oxidation (which depends on coupled mitochondrial oxygen consumption). Column v gives the overall sum of these partial P/O ratios, which are the same as column q in A. β-ox, β-oxidation; oxphos, oxidative phosphorylation. C, the total rate of ATP production, JATP production, is the sum of measurable extracellular rates (PPR and OCR) multiplied by the appropriate ATP/lactate ratio from A or P/O ratio from B.