Functional Human Beige Adipocytes From Induced Pluripotent Stem Cells
- PMID: 28270520
- PMCID: PMC5440013
- DOI: 10.2337/db16-1107
Functional Human Beige Adipocytes From Induced Pluripotent Stem Cells
Abstract
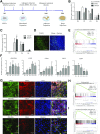
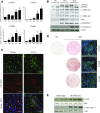
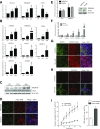
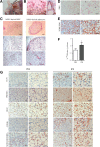
Activation of thermogenic beige adipocytes has recently emerged as a promising therapeutic target in obesity and diabetes. Relevant human models for beige adipocyte differentiation are essential to implement such therapeutic strategies. We report a straightforward and efficient protocol to generate functional human beige adipocytes from human induced pluripotent stem cells (hiPSCs). Without overexpression of exogenous adipogenic genes, our method recapitulates an adipogenic developmental pathway through successive mesodermal and adipogenic progenitor stages. hiPSC-derived adipocytes are insulin sensitive and display beige-specific markers and functional properties, including upregulation of thermogenic genes, increased mitochondrial content, and increased oxygen consumption upon activation with cAMP analogs. Engraftment of hiPSC-derived adipocytes in mice produces well-organized and vascularized adipose tissue, capable of β-adrenergic-responsive glucose uptake. Our model of human beige adipocyte development provides a new and scalable tool for disease modeling and therapeutic screening.
© 2017 by the American Diabetes Association.
Figures
Comment in
-
Adipose tissue: New route to functional human beige adipocytes.Nat Rev Endocrinol. 2017 May;13(5):251. doi: 10.1038/nrendo.2017.34. Epub 2017 Mar 24. Nat Rev Endocrinol. 2017. PMID: 28338661 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

