Phosphoproteins in extracellular vesicles as candidate markers for breast cancer
- PMID: 28270605
- PMCID: PMC5373352
- DOI: 10.1073/pnas.1618088114
Phosphoproteins in extracellular vesicles as candidate markers for breast cancer
Abstract
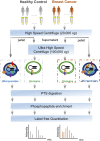
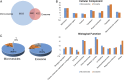
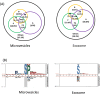
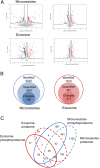
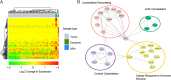
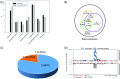
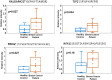
The state of protein phosphorylation can be a key determinant of cellular physiology such as early-stage cancer, but the development of phosphoproteins in biofluids for disease diagnosis remains elusive. Here we demonstrate a strategy to isolate and identify phosphoproteins in extracellular vesicles (EVs) from human plasma as potential markers to differentiate disease from healthy states. We identified close to 10,000 unique phosphopeptides in EVs isolated from small volumes of plasma samples. Using label-free quantitative phosphoproteomics, we identified 144 phosphoproteins in plasma EVs that are significantly higher in patients diagnosed with breast cancer compared with healthy controls. Several biomarkers were validated in individual patients using paralleled reaction monitoring for targeted quantitation. This study demonstrates that the development of phosphoproteins in plasma EV as disease biomarkers is highly feasible and may transform cancer screening and monitoring.
Keywords: biomarker; extracellular vesicles; mass spectrometry; phosphoproteins; proteomics.
Conflict of interest statement
A.B.I. and W.A.T. are cofounders of Tymora Analytical Operations.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

