Ablation of cytochrome P450 omega-hydroxylase 4A14 gene attenuates hepatic steatosis and fibrosis
- PMID: 28270609
- PMCID: PMC5373383
- DOI: 10.1073/pnas.1700172114
Ablation of cytochrome P450 omega-hydroxylase 4A14 gene attenuates hepatic steatosis and fibrosis
Erratum in
-
Correction for Zhang et al., Ablation of cytochrome P450 omega-hydroxylase 4A14 gene attenuates hepatic steatosis and fibrosis.Proc Natl Acad Sci U S A. 2023 Oct 17;120(42):e2315591120. doi: 10.1073/pnas.2315591120. Epub 2023 Oct 12. Proc Natl Acad Sci U S A. 2023. PMID: 37824533 Free PMC article. No abstract available.
Abstract
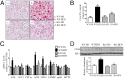
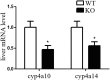
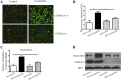
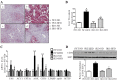
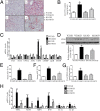
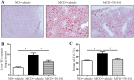
Nonalcoholic fatty liver disease (NAFLD) is characterized by simple hepatic steatosis (SS), nonalcoholic steatohepatitis (NASH), hepatic fibrosis, and cirrhosis. Dysregulated fatty acid metabolism in the liver plays a critical role in the pathogenesis of NAFLD. Cytochrome P450 omega-hydroxylase 4A14 (CYP4A14) is a homolog of human CYP4A hydroxylase that catalyzes omega-hydroxylation of medium-chain fatty acids and arachidonic acid in mice. The goal of this study was to determine the role of CYP4A14 in the development and the progression of NAFLD. Here, we showed that hepatic CYP4A expression was up-regulated in the livers of patients and three murine models of NAFLD. Adenovirus-mediated overexpression of CYP4A14 in the livers of C57BL/6 mice resulted in a fatty liver phenotype with a significant increase in hepatic fatty acid translocase (FAT/CD36) expression. In contrast, CYP4A14 gene-deficient mice fed a high-fat diet or a methionine and choline-deficient (MCD) diet exhibited attenuated liver lipid accumulation and reduced hepatic FAT/CD36 expression. In addition, hepatic inflammation and fibrosis was markedly ameliorated in MCD diet-fed CYP4A14-deficient mice. Collectively, CYP4A14 plays an important role in the pathogenesis of both SS and NASH and may represent a potential therapeutic target for the treatment of NAFLD.
Keywords: NAFLD; arachidonic acid; fatty acid; hepatic fibrosis; inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649–1657. - PubMed
-
- Musso G, Gambino R, Cassader M. Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD) Prog Lipid Res. 2009;48(1):1–26. - PubMed
-
- Hardwick JP. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem Pharmacol. 2008;75(12):2263–2275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

