Abscisic Acid Induces Resistance against Bamboo Mosaic Virus through Argonaute2 and 3
- PMID: 28270624
- PMCID: PMC5411131
- DOI: 10.1104/pp.16.00015
Abscisic Acid Induces Resistance against Bamboo Mosaic Virus through Argonaute2 and 3
Abstract
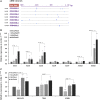
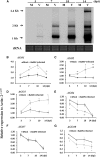
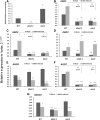
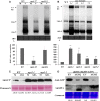
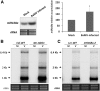
Plant resistance to pathogens is tuned by defense-related hormones. Of these, abscisic acid (ABA) is well documented to moderate resistance against fungi and bacteria. However, ABA's contribution to resistance against viruses is pleiotropic. ABA affects callose deposition at plasmodesmata (therefore hindering the viral cell-to-cell movement), but here, we show that when callose synthase is down-regulated, ABA still induces resistance against infection with Bamboo mosaic virus (BaMV). By examining the potential connections between the ABA and RNA-silencing pathways in Arabidopsis (Arabidopsis thaliana), we showed that ABA regulates the expression of almost the whole ARGONAUTE (AGO) gene family, of which some are required for plant resistance against BaMV Our data show that BaMV infection and ABA treatment regulate the same set of AGOs, with positive effects on AGO1, AGO2, and AGO3, no effect on AGO7, and negative effects on AGO4 and AGO10 The BaMV-mediated regulation of AGO1, AGO2, and AGO3 is ABA dependent, because the accumulation of these AGOs in BaMV-infected ABA mutants did not reach the levels observed in infected wild-type plants. In addition, the AGO1-miR168a complex is dispensable for BaMV resistance, while AGO2 and AGO3 were important for ABA-mediated resistance. While most ago mutants showed increased susceptibility to BaMV infection (except ago10), ago1-27 showed reduced BaMV titers, which was attributed to the up-regulated levels of AGO2, AGO3, and AGO4 We have established that ABA regulates the expression of several members of the AGO family, and this regulation partially contributes to ABA-mediated resistance against BaMV These findings reveal another role for ABA in plants.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures



References
-
- Alamillo JM, Saénz P, García JA (2006) Salicylic acid-mediated and RNA-silencing defense mechanisms cooperate in the restriction of systemic spread of plum pox virus in tobacco. Plant J 48: 217–227 - PubMed
-
- Alazem M, Lin KY, Lin NS (2014) The abscisic acid pathway has multifaceted effects on the accumulation of Bamboo mosaic virus. Mol Plant Microbe Interact 27: 177–189 - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

