Borrelia burgdorferi Keeps Moving and Carries on: A Review of Borrelial Dissemination and Invasion
- PMID: 28270812
- PMCID: PMC5318424
- DOI: 10.3389/fimmu.2017.00114
Borrelia burgdorferi Keeps Moving and Carries on: A Review of Borrelial Dissemination and Invasion
Abstract
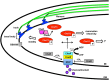
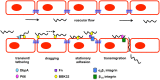
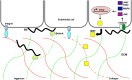
Borrelia burgdorferi is the etiological agent of Lyme disease, a multisystemic, multistage, inflammatory infection resulting in patients experiencing cardiac, neurological, and arthritic complications when not treated with antibiotics shortly after exposure. The spirochetal bacterium transmits through the Ixodes vector colonizing the dermis of a mammalian host prior to hematogenous dissemination and invasion of distal tissues all the while combating the immune response as it traverses through its pathogenic lifecycle. The innate immune response controls the borrelial burden in the dermis, but is unable to clear the infection and thereby prevent progression of disease. Dissemination in the mammalian host requires temporal regulation of virulence determinants to allow for vascular interactions, invasion, and colonization of distal tissues. Virulence determinants and/or adhesins are highly heterogenetic among environmental B. burgdorferi strains with particular genotypes being associated with the ability to disseminate to specific tissues and the severity of disease, but fail to generate cross-protective immunity between borrelial strains. The unique motility of B. burgdorferi rendered by the endoflagella serves a vital function for dissemination and protection from immune recognition. Progress has been made toward understanding the chemotactic regulation coordinating the activity of the two polar localized flagellar motors and their role in borrelial virulence, but this regulation is not yet fully understood. Distinct states of motility allow for dynamic interactions between several B. burgdorferi adhesins and host targets that play roles in transendothelial migration. Transmigration across endothelial and blood-brain barriers allows for the invasion of tissues and elicits localized immune responses. The invasive nature of B. burgdorferi is lacking in proactive mechanisms to modulate disease, such as secretion systems and toxins, but recent work has shown degradation of host extracellular matrices by B. burgdorferi contributes to the invasive capabilities of the pathogen. Additionally, B. burgdorferi may use invasion of eukaryotic cells for immune evasion and protection against environmental stresses. This review provides an overview of B. burgdorferi mechanisms for dissemination and invasion in the mammalian host, which are essential for pathogenesis and the development of persistent infection.
Keywords: Borrelia burgdorferi; Lyme disease; chemotaxis; dissemination; invasion; motility; protease; vascular interaction.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

