Optimized sensitivity of Silicon-on-Insulator (SOI) strip waveguide resonator sensor
- PMID: 28270963
- PMCID: PMC5330585
- DOI: 10.1364/BOE.8.000500
Optimized sensitivity of Silicon-on-Insulator (SOI) strip waveguide resonator sensor
Abstract
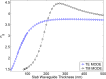
Evanescent field sensors have shown promise for biological sensing applications. In particular, Silicon-on-Insulator (SOI)-nano-photonic based resonator sensors have many advantages for lab-on-chip diagnostics, including high sensitivity for molecular detection and compatibility with CMOS foundries for high volume manufacturing. We have investigated the optimum design parameters within the fabrication constraints of Multi-Project Wafer (MPW) foundries that result in the highest sensitivity for a resonator sensor. We have demonstrated the optimum waveguide thickness needed to achieve the maximum bulk sensitivity with SOI-based resonator sensors to be 165 nm using the quasi-TM guided mode. The closest thickness offered by MPW foundry services is 150 nm. Therefore, resonators with 150 nm thick silicon waveguides were fabricated resulting in sensitivities as high as 270 nm/RIU, whereas a similar resonator sensor with a 220 nm thick waveguide demonstrated sensitivities of approximately 200 nm/RIU.
Keywords: (140.4780) Optical resonators; (280.1415) Biological sensing and sensors.
Figures




References
-
- Tomita M. R., Russ L. S., Sridhar R., Naughton B. J., “Smart home with healthcare technologies for community-dwelling older adults,” Available as on 13th of March (2012).
-
- Talebi Fard S., Grist S. M., Donzella V., Schmidt S. A., Flueckiger J., Wang X., Shi W., Millspaugh A., Webb M., Ratner D. M., Cheung K. C., Chrostowski L., “Label-free silicon photonic biosensors for use in clinical diagnostics,” Proc. SPIE, Silicon Photonics VIII 8629, 862909 (2013). 10.1117/12.2005832 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
