Measurement of dynamic cell-induced 3D displacement fields in vitro for traction force optical coherence microscopy
- PMID: 28271010
- PMCID: PMC5330596
- DOI: 10.1364/BOE.8.001152
Measurement of dynamic cell-induced 3D displacement fields in vitro for traction force optical coherence microscopy
Abstract
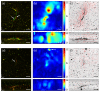
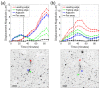
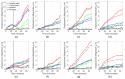
Traction force microscopy (TFM) is a method used to study the forces exerted by cells as they sense and interact with their environment. Cell forces play a role in processes that take place over a wide range of spatiotemporal scales, and so it is desirable that TFM makes use of imaging modalities that can effectively capture the dynamics associated with these processes. To date, confocal microscopy has been the imaging modality of choice to perform TFM in 3D settings, although multiple factors limit its spatiotemporal coverage. We propose traction force optical coherence microscopy (TF-OCM) as a novel technique that may offer enhanced spatial coverage and temporal sampling compared to current methods used for volumetric TFM studies. Reconstructed volumetric OCM data sets were used to compute time-lapse extracellular matrix deformations resulting from cell forces in 3D culture. These matrix deformations revealed clear differences that can be attributed to the dynamic forces exerted by normal versus contractility-inhibited NIH-3T3 fibroblasts embedded within 3D Matrigel matrices. Our results are the first step toward the realization of 3D TF-OCM, and they highlight the potential use of OCM as a platform for advancing cell mechanics research.
Keywords: (100.6950) Tomographic image processing; (170.1530) Cell analysis; (170.4500) Optical coherence tomography; (170.6900) Three-dimensional microscopy.
Figures


Similar articles
-
Quantitative reconstruction of time-varying 3D cell forces with traction force optical coherence microscopy.Sci Rep. 2019 Mar 11;9(1):4086. doi: 10.1038/s41598-019-40608-4. Sci Rep. 2019. PMID: 30858424 Free PMC article.
-
Computational 4D-OCM for label-free imaging of collective cell invasion and force-mediated deformations in collagen.Sci Rep. 2021 Feb 2;11(1):2814. doi: 10.1038/s41598-021-81470-7. Sci Rep. 2021. PMID: 33531512 Free PMC article.
-
Advanced in silico validation framework for three-dimensional traction force microscopy and application to an in vitro model of sprouting angiogenesis.Acta Biomater. 2021 May;126:326-338. doi: 10.1016/j.actbio.2021.03.014. Epub 2021 Mar 15. Acta Biomater. 2021. PMID: 33737201
-
Toward single cell traction microscopy within 3D collagen matrices.Exp Cell Res. 2013 Oct 1;319(16):2396-408. doi: 10.1016/j.yexcr.2013.06.009. Epub 2013 Jun 25. Exp Cell Res. 2013. PMID: 23806281 Free PMC article. Review.
-
2.5D Traction Force Microscopy: Imaging three-dimensional cell forces at interfaces and biological applications.Int J Biochem Cell Biol. 2023 Aug;161:106432. doi: 10.1016/j.biocel.2023.106432. Epub 2023 Jun 7. Int J Biochem Cell Biol. 2023. PMID: 37290687 Review.
Cited by
-
High-resolution assessment of multidimensional cellular mechanics using label-free refractive-index traction force microscopy.Commun Biol. 2024 Jan 20;7(1):115. doi: 10.1038/s42003-024-05788-4. Commun Biol. 2024. PMID: 38245624 Free PMC article.
-
Live-single-cell phenotypic cancer biomarkers-future role in precision oncology?NPJ Precis Oncol. 2017 Jun 15;1(1):21. doi: 10.1038/s41698-017-0025-y. eCollection 2017. NPJ Precis Oncol. 2017. PMID: 29872705 Free PMC article. Review.
-
A Review of Single-Cell Adhesion Force Kinetics and Applications.Cells. 2021 Mar 5;10(3):577. doi: 10.3390/cells10030577. Cells. 2021. PMID: 33808043 Free PMC article. Review.
-
Volumetric optical coherence microscopy with a high space-bandwidth-time product enabled by hybrid adaptive optics.Biomed Opt Express. 2018 Jun 15;9(7):3137-3152. doi: 10.1364/BOE.9.003137. eCollection 2018 Jul 1. Biomed Opt Express. 2018. PMID: 29984088 Free PMC article.
-
Optical coherence microscopy with a split-spectrum image reconstruction method for temporal-dynamics contrast-based imaging of intracellular motility.Biomed Opt Express. 2023 Jan 4;14(2):577-592. doi: 10.1364/BOE.478264. eCollection 2023 Feb 1. Biomed Opt Express. 2023. PMID: 36874497 Free PMC article.
References
-
- Seo B. R., Bhardwaj P., Choi S., Gonzalez J., Andresen Eguiluz R. C., Wang K., Mohanan S., Morris P. G., Du B., Zhou X. K., Vahdat L. T., Verma A., Elemento O., Hudis C. A., Williams R. M., Gourdon D., Dannenberg A. J., Fischbach C., “Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis,” Sci. Transl. Med. 7(301), 301ra130 (2015).10.1126/scitranslmed.3010467 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
