Deep feature learning for automatic tissue classification of coronary artery using optical coherence tomography
- PMID: 28271012
- PMCID: PMC5330543
- DOI: 10.1364/BOE.8.001203
Deep feature learning for automatic tissue classification of coronary artery using optical coherence tomography
Abstract
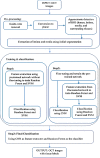
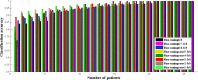
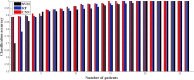
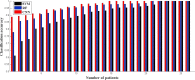
Kawasaki disease (KD) is an acute childhood disease complicated by coronary artery aneurysms, intima thickening, thrombi, stenosis, lamellar calcifications, and disappearance of the media border. Automatic classification of the coronary artery layers (intima, media, and scar features) is important for analyzing optical coherence tomography (OCT) images recorded in pediatric patients. OCT has been known as an intracoronary imaging modality using near-infrared light which has recently been used to image the inner coronary artery tissues of pediatric patients, providing high spatial resolution (ranging from 10 to 20 μm). This study aims to develop a robust and fully automated tissue classification method by using the convolutional neural networks (CNNs) as feature extractor and comparing the predictions of three state-of-the-art classifiers, CNN, random forest (RF), and support vector machine (SVM). The results show the robustness of CNN as the feature extractor and random forest as the classifier with classification rate up to 96%, especially to characterize the second layer of coronary arteries (media), which is a very thin layer and it is challenging to be recognized and specified from other tissues.
Keywords: (100.0100) Image processing; (100.2960) Image analysis; (100.4996) Pattern recognition, neural networks; (110.0110) Imaging systems; (110.2960) Image analysis; (110.4500) Optical coherence tomography.
Figures



References
-
- Dionne A., Ibrahim R., Gebhard C., Bakloul M., Selly J.-B., Leye M., Déry J., Lapierre C., Girard P., Fournier A., Dahdah N., “Coronary wall structural changes in patients with kawasaki disease: new insights from optical coherence tomography (oct),” J. Am. Heart Assoc. 4, e001939 (2015). 10.1161/JAHA.115.001939 - DOI - PMC - PubMed
-
- Preim B., Bartz D., Visualization in Medicine: Theory, Algorithms, and Applications (Morgan Kaufmann, 2007).
LinkOut - more resources
Full Text Sources
Other Literature Sources
