Characterization of the peripheral neuropathy associated with brentuximab vedotin treatment of Mycosis Fungoides and Sézary Syndrome
- PMID: 28271282
- PMCID: PMC5955867
- DOI: 10.1007/s11060-017-2389-9
Characterization of the peripheral neuropathy associated with brentuximab vedotin treatment of Mycosis Fungoides and Sézary Syndrome
Abstract
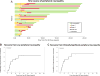
Chemotherapy-induced peripheral neuropathy (CIPN) is common, frequently limits chemotherapy dosing, and negatively impacts quality of life. The National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE), version 4.0, and the Total Neuropathy Score clinical version (TNSc) are both validated scores to quantify peripheral neuropathy (PN), with the TNSc being more sensitive to clinical changes. Mycosis fungoides and Sézary syndrome (MF/SS) are characterized by a chronic course, where current therapies are generally non-curative and treatment toxicities have the potential for significant lasting effects. Brentuximab vedotin (BV) is an antibody-drug-conjugate composed of an anti-CD30 monoclonal antibody linked to the microtubule-disrupting agent, monomethyl auristatin E, with a known associated CIPN. In our phase II clinical trial of BV in MF/SS, 25 (69%) of 36 patients developed PN, with 18 (50%) developing Clinically Significant PN, CTCAE v4.0 grade 2 or higher. The median time to grade 2 PN was 15 weeks (range 0.4-48) after the initial dose. By Kaplan-Meier calculation, the median time to improvement from Clinically Significant PN was 30 weeks from the last BV dose. Seventy-four percent had improvement by 24 months. We found that TNSc scores significantly correlated with CTCAE grade, with Spearman correlation coefficient 0.68 (p < 0.001). By logistic regression, for each 100 mg increase in BV total dose, the likelihood of developing Clinically Significant PN increased by 23% (95% CI 4-46%). Improved monitoring of CIPN associated with BV is of paramount importance in the MF/SS population.
Keywords: Brentuximab vedotin; Chemotherapy-induced peripheral neuropathy; Mycosis fungoides; Neuropathy; Sézary syndrome; Total neuropathy score.
Conflict of interest statement
Author Y Kim has received research grants from Seattle Genetics. All remaining authors declare they have no conflict of interest to report.
Figures


Comment in
-
Brentuximab vedotin-induced peripheral neuropathy: looking at microtubules.J Neurooncol. 2018 May;137(3):665-666. doi: 10.1007/s11060-018-2743-6. Epub 2018 Jan 9. J Neurooncol. 2018. PMID: 29318508 No abstract available.
Similar articles
-
Phase II Investigator-Initiated Study of Brentuximab Vedotin in Mycosis Fungoides and Sézary Syndrome With Variable CD30 Expression Level: A Multi-Institution Collaborative Project.J Clin Oncol. 2015 Nov 10;33(32):3750-8. doi: 10.1200/JCO.2014.60.3969. Epub 2015 Jul 20. J Clin Oncol. 2015. PMID: 26195720 Free PMC article. Clinical Trial.
-
Results of a Phase II Trial of Brentuximab Vedotin for CD30+ Cutaneous T-Cell Lymphoma and Lymphomatoid Papulosis.J Clin Oncol. 2015 Nov 10;33(32):3759-65. doi: 10.1200/JCO.2014.60.3787. Epub 2015 Aug 10. J Clin Oncol. 2015. PMID: 26261247 Free PMC article. Clinical Trial.
-
Phase I/II clinical trial of brentuximab vedotin for pretreated Japanese patients with CD30-positive cutaneous T-cell lymphoma.J Dermatol. 2024 Aug;51(8):1037-1049. doi: 10.1111/1346-8138.17324. Epub 2024 Jun 14. J Dermatol. 2024. PMID: 38874430 Free PMC article. Clinical Trial.
-
Brentuximab vedotin in CD30+ primary cutaneous T-cell lymphomas: a review and analysis of existing data.Int J Dermatol. 2017 Dec;56(12):1400-1405. doi: 10.1111/ijd.13696. Epub 2017 Aug 1. Int J Dermatol. 2017. PMID: 28762479 Review.
-
Current systemic therapeutic options for advanced mycosis fungoides and Sézary syndrome.Leuk Lymphoma. 2018 Mar;59(3):562-577. doi: 10.1080/10428194.2017.1347650. Epub 2018 Jan 8. Leuk Lymphoma. 2018. PMID: 29308723 Review.
Cited by
-
Brentuximab vedotin-induced peripheral neuropathy: looking at microtubules.J Neurooncol. 2018 May;137(3):665-666. doi: 10.1007/s11060-018-2743-6. Epub 2018 Jan 9. J Neurooncol. 2018. PMID: 29318508 No abstract available.
-
Cardiac toxicity of brentuximab vedotin: a real-word disproportionality analysis of the FDA Adverse Event Reporting System (FAERS) database.Naunyn Schmiedebergs Arch Pharmacol. 2024 Jul;397(7):5253-5264. doi: 10.1007/s00210-024-02955-6. Epub 2024 Jan 25. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38270617
-
Histopathological Markers for Target Therapies in Primary Cutaneous Lymphomas.Cells. 2023 Nov 20;12(22):2656. doi: 10.3390/cells12222656. Cells. 2023. PMID: 37998391 Free PMC article. Review.
-
Chemotherapy and peripheral neuropathy.Neurol Sci. 2021 Oct;42(10):4109-4121. doi: 10.1007/s10072-021-05576-6. Epub 2021 Aug 26. Neurol Sci. 2021. PMID: 34436727 Review.
-
Electrophysiological Studies in Combination With Interim-Positron Emission Tomography Scan for Prevention of Severe Brentuximab-Vedotin-Induced Neurotoxicity.Eur J Haematol. 2025 May;114(5):802-811. doi: 10.1111/ejh.14384. Epub 2025 Jan 7. Eur J Haematol. 2025. PMID: 39777790 Free PMC article.
References
-
- Hershman DL, Weimer LH, Wang A, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2010;125(3):767–774. doi: 10.1007/s10549-010-1278-0. - DOI - PubMed
-
- DeAngelis LM, Posner JB. Neurologic complications of cancer. 2nd. Oxford University Press; Oxford: 2009.
-
- Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53(8):1660–1664. http://www.neurology.org.laneproxy.stanford.edu/content/53/8/1660.full#r.... - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

