Patient-important outcomes in randomized controlled trials in critically ill patients: a systematic review
- PMID: 28271450
- PMCID: PMC5340787
- DOI: 10.1186/s13613-017-0243-z
Patient-important outcomes in randomized controlled trials in critically ill patients: a systematic review
Abstract
Background: Intensivists' clinical decision making pursues two main goals for patients: to decrease mortality and to improve quality of life and functional status in survivors. Patient-important outcomes are gaining wide acceptance in most fields of clinical research. We sought to systematically review how well patient-important outcomes are reported in published randomized controlled trials (RCTs) in critically ill patients.
Methods: Literature search was conducted to identify eligible trials indexed from January to December 2013. Articles were eligible if they reported an RCT involving critically ill adult patients. We excluded phase II, pilot and physiological crossover studies. We assessed study characteristics. All primary and secondary outcomes were collected, described and classified using six categories of outcomes including patient-important outcomes (involving mortality at any time on the one hand and quality of life, functional/cognitive/neurological outcomes assessed after ICU discharge on the other).
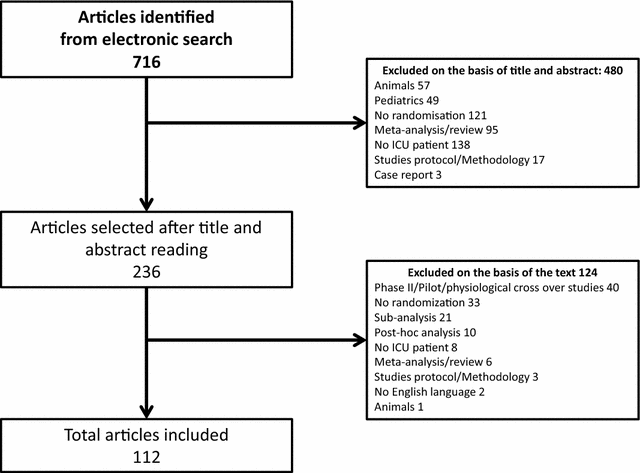
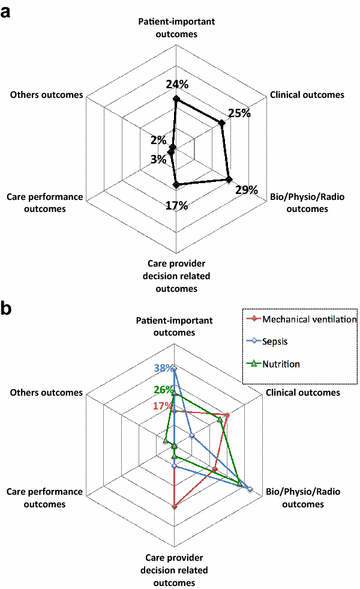
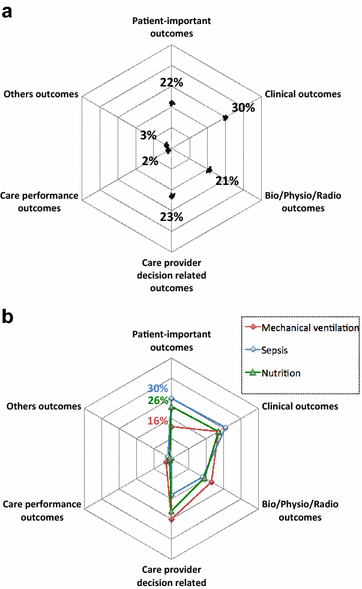
Results: Of the 716 articles retrieved in 2013, 112 RCTs met the inclusion criteria. Most common topics were mechanical ventilation (27%), sepsis (19%) and nutrition (17%). Among the 112 primary outcomes, 27 (24%) were patient-important outcomes (mainly mortality, 21/27) but only six (5%) were patient-important outcomes besides mortality assessed after ICU discharge (functional disability = 4; quality of life = 2). Among the 598 secondary outcomes, 133 (22%) were patient-important outcomes (mainly mortality, 92/133) but only 41 (7%) were patient-important outcomes besides mortality assessed after ICU discharge (quality of life = 20, functional disability = 14; neurological/cognitive performance = 5; handicap = 1; post-traumatic stress = 1). Seventy-three RCTs (65%) reported at least one patient-important outcome but only 11 (10%) reported at least one patient-important outcome besides mortality assessed after ICU discharge.
Conclusion: Patient-important outcomes are rarely primary outcomes in RCTs in critically ill patients published in 2013. Among them, mortality accounted for the majority. We promote the use of patient-important outcomes in critical care trials.
Keywords: Critical care; Patient-important outcome; Quality of life.
Figures
References
-
- Guyatt G, Montori V, Devereaux PJ, Schünemann H, Bhandari M. Patients at the center: in our practice, and in our use of language. ACP J Club. 2004;140(1):A11–A12. - PubMed
-
- Brower R, Matthay M, Moris A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

