Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents
- PMID: 28271521
- PMCID: PMC5849458
- DOI: 10.1111/apt.14021
Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents
Abstract
Background: Highly effective direct antiviral agents (DAAs) for hepatitis C virus (HCV) were introduced recently. Their utilisation has been limited by high cost and low access to care.
Aim: To describe the effect of DAAs on HCV treatment and cure rates in the United States Veterans Affairs (VA) national healthcare system.
Methods: We identified all HCV antiviral treatment regimens initiated from 1 January 1999 to 31 December 2015 (n = 105 369) in the VA national healthcare system, and determined if they resulted in sustained virological response (SVR).
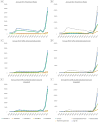
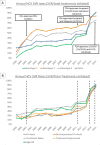
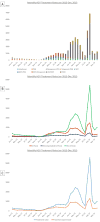
Results: HCV antiviral treatment rates were low (1981-6679 treatments/year) in the interferon era (1999-2010). The introduction of simeprevir and sofosbuvir in 2013 and ledipasvir/sofosbuvir and paritaprevir/ombitasvir/ritonavir/dasabuvir in 2014 were followed by increases in annual treatment rates to 9180 in 2014 and 31 028 in 2015. The number of patients achieving SVR was 1313 in 2010, the last year of the interferon era, and increased 5.6-fold to 7377 in 2014 and 21-fold to 28 084 in 2015. The proportion of treated patients who achieved SVR increased from 19.2% in 1999 and 36.0% in 2010 to 90.5% in 2015. Within 2015, monthly treatment rates ranged from 727 in July to 6868 in September correlating with the availability of funds for DAAs.
Conclusions: DAAs resulted in a 21-fold increase in the number of patients achieving HCV cure. Treatment rates in 2015 were limited primarily by the availability of funds. Further increases in funding and cost reductions of DAAs in 2016 suggest that the VA could cure the majority of HCV-infected Veterans in VA care within the next few years.
© 2017 John Wiley & Sons Ltd.
Conflict of interest statement
None
Figures

References
-
- Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98. - PubMed
-
- Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93. - PubMed
-
- Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–88. - PubMed
-
- Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–603. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
