Multi-layered inhibition of Streptomyces development: BldO is a dedicated repressor of whiB
- PMID: 28271577
- PMCID: PMC5485038
- DOI: 10.1111/mmi.13663
Multi-layered inhibition of Streptomyces development: BldO is a dedicated repressor of whiB
Abstract
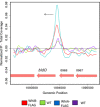
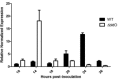
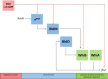
BldD-(c-di-GMP) sits on top of the regulatory network that controls differentiation in Streptomyces, repressing a large regulon of developmental genes when the bacteria are growing vegetatively. In this way, BldD functions as an inhibitor that blocks the initiation of sporulation. Here, we report the identification and characterisation of BldO, an additional developmental repressor that acts to sustain vegetative growth and prevent entry into sporulation. However, unlike the pleiotropic regulator BldD, we show that BldO functions as the dedicated repressor of a single key target gene, whiB, and that deletion of bldO or constitutive expression of whiB is sufficient to induce precocious hypersporulation.
© 2017 The Authors Molecular Microbiology Published by John Wiley & Sons Ltd.
Figures






References
-
- Bibb, M.J. , Domonkos, A. , Chandra, G. , and Buttner, M.J. (2012) Expression of the chaplin and rodlin hydrophobic sheath proteins in Streptomyces venezuelae is controlled by σBldN and a cognate anti‐sigma factor, RsbN. Mol Microbiol 84: 1033–1049. - PubMed
-
- Brown, N.L. , Stoyanov, J.V. , Kidd, S.P. , and Hobman, J.L. (2003) The MerR family of transcriptional regulators. FEMS Microbiol Rev 27: 145–163. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

