Multidomain proteins under force
- PMID: 28272024
- PMCID: PMC5533105
- DOI: 10.1088/1361-6528/aa655e
Multidomain proteins under force
Abstract
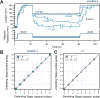
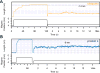
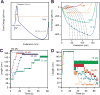
Advancements in single-molecule force spectroscopy techniques such as atomic force microscopy and magnetic tweezers allow investigation of how domain folding under force can play a physiological role. Combining these techniques with protein engineering and HaloTag covalent attachment, we investigate similarities and differences between four model proteins: I10 and I91-two immunoglobulin-like domains from the muscle protein titin, and two α + β fold proteins-ubiquitin and protein L. These proteins show a different mechanical response and have unique extensions under force. Remarkably, when normalized to their contour length, the size of the unfolding and refolding steps as a function of force reduces to a single master curve. This curve can be described using standard models of polymer elasticity, explaining the entropic nature of the measured steps. We further validate our measurements with a simple energy landscape model, which combines protein folding with polymer physics and accounts for the complex nature of tandem domains under force. This model can become a useful tool to help in deciphering the complexity of multidomain proteins operating under force.
Figures
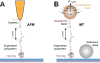


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
