Brief Report: Long-Term (96-Week) Efficacy and Safety After Switching From Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in HIV-Infected, Virologically Suppressed Adults
- PMID: 28272164
- PMCID: PMC5427981
- DOI: 10.1097/QAI.0000000000001344
Brief Report: Long-Term (96-Week) Efficacy and Safety After Switching From Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in HIV-Infected, Virologically Suppressed Adults
Abstract
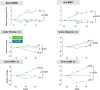
In a double-blind, phase 3 trial, 663 HIV-infected, virologically suppressed adults were randomized to switch to tenofovir alafenamide (TAF; n = 333) vs. remain on tenofovir disoproxil fumarate (TDF; n = 330), each coformulated with emtricitabine (FTC), while continuing their third agent (boosted protease inhibitor or unboosted third agent). At week 96, 88.6% on FTC/TAF and 89.1% on FTC/TDF had HIV-1 RNA <50 copies per milliliter [adjusted difference -0.5% (95% confidence interval: -5.3 to 4.4%)]. Proteinuria, albuminuria, proximal renal tubular function, and bone mineral density improved after switching to TAF- from TDF-containing regimens. These longer-term data support FTC/TAF as a safe, well-tolerated, and durable nucleotide reverse transcriptase inhibitor backbone.
Conflict of interest statement
C.O. has received honoraria for lectures and for advisory boards and educational grants, travel scholarships and research grants from Gilead, AbbVie, Merck Sharp and Dome, Bristol-Myers Squibb, ViiV, Janssen, Boehringer Ingelheim, Abbott, and GlaxoSmithKline. L.S. reports personal fees from ViiV, Bristol-Myers Squibb, Gilead, and the AIDS International Education Project. V-AF reports grants from Agence Nationale de Recherche sur le Sida (ANRS) and conference fees from Gilead. E.D. is a consultant/advisor for Bristol-Myers Squibb, Gilead, Janssen, Teva, and ViiV and received research support from Gilead, Merck, and ViiV. K.H. has received research support from Gilead Sciences and ViiV Healthcare. J.S.-B. reports grant support from NIAID/NIDA, Merck, and Gilead, personal fees from Merck, Bristol-Myers Squibb, ViiV, and Gilead, and other support from Gilead. N.B. has received consultancy fees, speaking honoraria, and research support from Abbott, GlaxoSmithKline, and Tiobtec. M.Y., M.E.A., SF, A.C., and M.S.R. are employees of Gilead and hold stock interest in the company. All other authors declare no conflicts of interests.
Figures
References
-
- Hall AM, Hendry BM, Nitsch D, et al. Tenofovir-associated kidney toxicity in HIV-infected patients: a review of the evidence. Am J Kidney Dis. 2011;57:773–780. - PubMed
-
- Gupta SK. Tenofovir-associated Fanconi syndrome: review of the FDA adverse event reporting system. AIDS Patient Care STDS. 2008;22:99–103. - PubMed
-
- Post FA, Moyle GJ, Stellbrink HJ, et al. Randomized comparison of renal effects, efficacy, and safety with once-daily abacavir/lamivudine versus tenofovir/emtricitabine, administered with efavirenz, in antiretroviral naive, HIV-1-infected adults: 48-week results from the ASSERT study. J Acquir Immune Defic Syndr. 2010;55:49–57. - PubMed
-
- Stellbrink HJ, Orkin C, Arribas JR, et al. Comparison of changes in bone density and turnover with abacavir–lamivudine versus tenofovir–emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis. 2010;51:963–972. - PubMed
-
- Schafer JJ, Manlangit K, Squires KE. Bone health and human immunodeficiency virus infection. Pharmacotherapy. 2013;33:665–682. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical