Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy
- PMID: 28272303
- PMCID: PMC5372585
- DOI: 10.3390/ijms18030569
Effects of Silver Nanoparticles on Multiple Drug-Resistant Strains of Staphylococcus aureus and Pseudomonas aeruginosa from Mastitis-Infected Goats: An Alternative Approach for Antimicrobial Therapy
Abstract
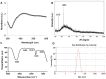
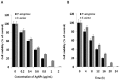
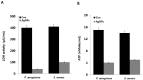
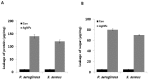
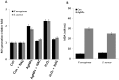
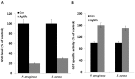
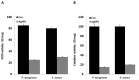
Recently, silver nanoparticles (AgNPs) have been widely used in various applications as antimicrobial agents, anticancer, diagnostics, biomarkers, cell labels, and drug delivery systems for the treatment of various diseases. Microorganisms generally acquire resistance to antibiotics through the course of antibacterial therapy. Multi-drug resistance (MDR) has become a growing problem in the treatment of infectious diseases, and the widespread use of broad-spectrum antibiotics has resulted in the development of antibiotic resistance by numerous human and animal bacterial pathogens. As a result, an increasing number of microorganisms are resistant to multiple antibiotics causing continuing economic losses in dairy farming. Therefore, there is an urgent need for the development of alternative, cost-effective, and efficient antimicrobial agents that overcome antimicrobial resistance. Here, AgNPs synthesized using the bio-molecule quercetin were characterized using various analytical techniques. The synthesized AgNPs were highly spherical in shape and had an average size of 11 nm. We evaluated the efficacy of synthesized AgNPs against two MDR pathogenic bacteria, namely, Pseudomonas aeruginosa and Staphylococcus aureus, which were isolated from milk samples produced by mastitis-infected goats. The minimum inhibitory concentrations (MICs) of AgNPs against P. aeruginosa and S. aureus were found to be 1 and 2 μg/mL, respectively. Our findings suggest that AgNPs exert antibacterial effects in a dose- and time-dependent manner. Results from the present study demonstrate that the antibacterial activity of AgNPs is due to the generation of reactive oxygen species (ROS), malondialdehyde (MDA), and leakage of proteins and sugars in bacterial cells. Results of the present study showed that AgNP-treated bacteria had significantly lower lactate dehydrogenase activity (LDH) and lower adenosine triphosphate (ATP) levels compared to the control. Furthermore, AgNP-treated bacteria showed downregulated expression of glutathione (GSH), upregulation of glutathione S-transferase (GST), and downregulation of both superoxide dismutase (SOD) and catalase (CAT). These physiological and biochemical measurements were consistently observed in AgNP-treated bacteria, thereby suggesting that AgNPs can induce bacterial cell death. Thus, the above results represent conclusive findings on the mechanism of action of AgNPs against different types of bacteria. This study also demonstrates the promising use of nanoparticles as antibacterial agents for use in the biotechnology and biomedical industry. Furthermore, this study is the first to propose the mode of action of AgNPs against MDR pathogens isolated from goats infected with subclinical mastitis.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; metabolic activity; multiple drug resistance; oxidative stress; silver nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Oliszewski R., Núñez D.K.M., González S., Oliver G. β-Glucuronidase method to determine mastitis levels in goat milk. Methods Mol. Biol. 2004;268:475–479. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

