Ornithine Aminotransferase, an Important Glutamate-Metabolizing Enzyme at the Crossroads of Multiple Metabolic Pathways
- PMID: 28272331
- PMCID: PMC5372011
- DOI: 10.3390/biology6010018
Ornithine Aminotransferase, an Important Glutamate-Metabolizing Enzyme at the Crossroads of Multiple Metabolic Pathways
Abstract
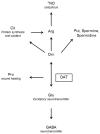
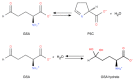
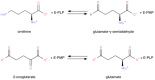
Ornithine δ-aminotransferase (OAT, E.C. 2.6.1.13) catalyzes the transfer of the δ-amino group from ornithine (Orn) to α-ketoglutarate (aKG), yielding glutamate-5-semialdehyde and glutamate (Glu), and vice versa. In mammals, OAT is a mitochondrial enzyme, mainly located in the liver, intestine, brain, and kidney. In general, OAT serves to form glutamate from ornithine, with the notable exception of the intestine, where citrulline (Cit) or arginine (Arg) are end products. Its main function is to control the production of signaling molecules and mediators, such as Glu itself, Cit, GABA, and aliphatic polyamines. It is also involved in proline (Pro) synthesis. Deficiency in OAT causes gyrate atrophy, a rare but serious inherited disease, a further measure of the importance of this enzyme.
Keywords: glutamate; gyrate atrophy; ornithine; ornithine aminotransferase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


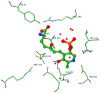


References
-
- Yasuo W. The Glutamate Crossway. In: Cynober L.A., editor. Metabolic and Therapeutic Aspects of Amino Acids in Clinical Nutrition. CRC Press; Boca Raton, FL, USA: 2004. pp. 135–152.
-
- Morris S.M. Arginine: Beyond protein. Am. J. Clin. Nutr. 2006;83:508S–512S. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

