Hydrogen Atomic Positions of O-H···O Hydrogen Bonds in Solution and in the Solid State: The Synergy of Quantum Chemical Calculations with ¹H-NMR Chemical Shifts and X-ray Diffraction Methods
- PMID: 28272366
- PMCID: PMC6155303
- DOI: 10.3390/molecules22030415
Hydrogen Atomic Positions of O-H···O Hydrogen Bonds in Solution and in the Solid State: The Synergy of Quantum Chemical Calculations with ¹H-NMR Chemical Shifts and X-ray Diffraction Methods
Abstract
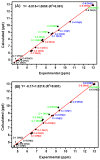
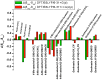
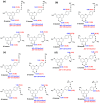
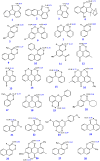
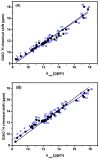
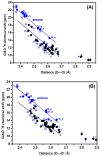
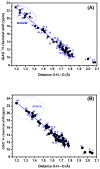
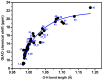
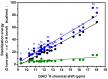
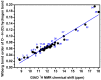
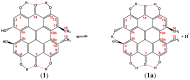
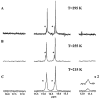
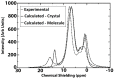
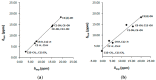
The exact knowledge of hydrogen atomic positions of O-H···O hydrogen bonds in solution and in the solid state has been a major challenge in structural and physical organic chemistry. The objective of this review article is to summarize recent developments in the refinement of labile hydrogen positions with the use of: (i) density functional theory (DFT) calculations after a structure has been determined by X-ray from single crystals or from powders; (ii) ¹H-NMR chemical shifts as constraints in DFT calculations, and (iii) use of root-mean-square deviation between experimentally determined and DFT calculated ¹H-NMR chemical shifts considering the great sensitivity of ¹H-NMR shielding to hydrogen bonding properties.
Keywords: DFT; NMR; X-ray diffraction; chemical shifts; hydrogen bonding.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

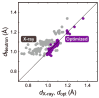

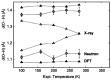
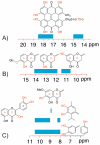



References
-
- Jeffrey G.A., Saenger S.W. Hydrogen Bonding in Biological Structures. Springer Verlag; Berlin, Germany: 1991.
-
- Jeffrey G.A. An Introduction to Hydrogen Bonding. Oxford University Press; New York, NY, USA: 1997.
-
- Scheider S. Hydrogen Bonding: A Theoretical Perspective. Oxford University Press; New York, NY, USA: 1997.
-
- Alkorta I., Rozas I., Elguero J. Non-conventional hydrogen bonds. Chem. Soc. Rev. 1998;27:163–170. doi: 10.1039/a827163z. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

