Regulation of DNA Replication through Natural Impediments in the Eukaryotic Genome
- PMID: 28272375
- PMCID: PMC5368702
- DOI: 10.3390/genes8030098
Regulation of DNA Replication through Natural Impediments in the Eukaryotic Genome
Abstract
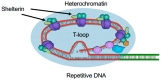
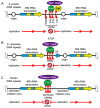
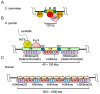
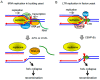
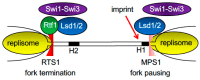
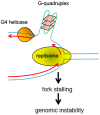
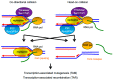
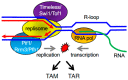
All living organisms need to duplicate their genetic information while protecting it from unwanted mutations, which can lead to genetic disorders and cancer development. Inaccuracies during DNA replication are the major cause of genomic instability, as replication forks are prone to stalling and collapse, resulting in DNA damage. The presence of exogenous DNA damaging agents as well as endogenous difficult-to-replicate DNA regions containing DNA-protein complexes, repetitive DNA, secondary DNA structures, or transcribing RNA polymerases, increases the risk of genomic instability and thus threatens cell survival. Therefore, understanding the cellular mechanisms required to preserve the genetic information during S phase is of paramount importance. In this review, we will discuss our current understanding of how cells cope with these natural impediments in order to prevent DNA damage and genomic instability during DNA replication.
Keywords: DNA replication; difficult‐to‐replicate; replication fork; replication machinery; replisome; DNA damage; genomic instability; natural impediments; repetitive DNA; secondary structures.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the organization and writing of the review manuscript.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

