Matching Dietary Amino Acid Balance to the In Silico-Translated Exome Optimizes Growth and Reproduction without Cost to Lifespan
- PMID: 28273481
- PMCID: PMC5355364
- DOI: 10.1016/j.cmet.2017.02.005
Matching Dietary Amino Acid Balance to the In Silico-Translated Exome Optimizes Growth and Reproduction without Cost to Lifespan
Erratum in
-
Matching Dietary Amino Acid Balance to the In Silico-Translated Exome Optimizes Growth and Reproduction without Cost to Lifespan.Cell Metab. 2017 May 2;25(5):1206. doi: 10.1016/j.cmet.2017.04.020. Cell Metab. 2017. PMID: 28467937 Free PMC article. No abstract available.
Abstract
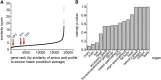
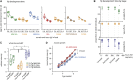
Balancing the quantity and quality of dietary protein relative to other nutrients is a key determinant of evolutionary fitness. A theoretical framework for defining a balanced diet would both reduce the enormous workload to optimize diets empirically and represent a breakthrough toward tailoring diets to the needs of consumers. Here, we report a simple and powerful in silico technique that uses the genome information of an organism to define its dietary amino acid requirements. We show for the fruit fly Drosophila melanogaster that such "exome-matched" diets are more satiating, enhance growth, and increase reproduction relative to non-matched diets. Thus, early life fitness traits can be enhanced at low levels of dietary amino acids that do not impose a cost to lifespan. Exome matching also enhanced mouse growth, indicating that it can be applied to other organisms whose genome sequence is known.
Keywords: Drosophila; amino acids; diet balance; dietary restriction; fitness; growth; lifespan; mouse; reproduction; trade-off.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures





Comment in
-
Feeding the Genome: In Silico Optimization of Dietary Amino Acid Composition.Cell Metab. 2017 Mar 7;25(3):486-488. doi: 10.1016/j.cmet.2017.02.015. Cell Metab. 2017. PMID: 28273468 Free PMC article.
References
-
- Chintapalli V.R., Wang J., Dow J.A. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007;39:715–720. - PubMed
-
- Cooper D.M. Food preferences of larval and adult Drosophila. Evolution. 1960;14:41–55.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

