Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity
- PMID: 28273482
- PMCID: PMC5345693
- DOI: 10.1016/j.cmet.2017.02.007
Orai1-Mediated Antimicrobial Secretion from Pancreatic Acini Shapes the Gut Microbiome and Regulates Gut Innate Immunity
Abstract
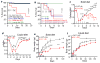
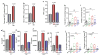
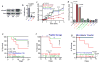
The gut microbiome participates in numerous physiologic functions and communicates intimately with the host immune system. Antimicrobial peptides are critical components of intestinal innate immunity. We report a prominent role for antimicrobials secreted by pancreatic acini in shaping the gut microbiome that is essential for intestinal innate immunity, barrier function, and survival. Deletion of the Ca2+ channel Orai1 in pancreatic acini of adult mice resulted in 60%-70% mortality within 3 weeks. Despite robust activation of the intestinal innate immune response, mice lacking acinar Orai1 exhibited intestinal bacterial outgrowth and dysbiosis, ultimately causing systemic translocation, inflammation, and death. While digestive enzyme supplementation was ineffective, treatments constraining bacterial outgrowth (purified liquid diet, broad-spectrum antibiotics) rescued survival, feeding, and weight gain. Pancreatic levels of cathelicidin-related antimicrobial peptide (CRAMP) were reduced, and supplement of synthetic CRAMP prevented intestinal disease. These findings reveal a critical role for antimicrobial pancreatic secretion in gut innate immunity.
Published by Elsevier Inc.
Figures




Comment in
-
Beyond Digestion: The Pancreas Shapes Intestinal Microbiota and Immunity.Cell Metab. 2017 Mar 7;25(3):495-496. doi: 10.1016/j.cmet.2017.02.018. Cell Metab. 2017. PMID: 28273472
-
Shaping the gut microbiome from the pancreas.Sci Signal. 2017 Mar 28;10(472):eaan3016. doi: 10.1126/scisignal.aan3016. Sci Signal. 2017. PMID: 28351952
References
-
- Biragyn A, Ruffini PA, Leifer CA, Klyushnenkova E, Shakhov A, Chertov O, Shirakawa AK, Farber JM, Segal DM, Oppenheim JJ, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298:1025–1029. - PubMed
-
- Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annual review of physiology. 2013;75:289–311. - PubMed
-
- Davis FM, Janoshazi A, Janardhan KS, Steinckwich N, D’Agostin DM, Petranka JG, Desai PN, Roberts-Thomson SJ, Bird GS, Tucker DK, et al. Essential role of Orai1 store-operated calcium channels in lactation. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:5827–5832. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

