Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse
- PMID: 28273875
- PMCID: PMC5372579
- DOI: 10.3390/ijms18030563
Characterization of ACE and ACE2 Expression within Different Organs of the NOD Mouse
Abstract
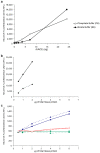
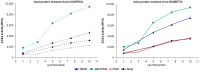
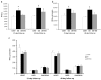
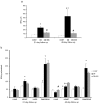
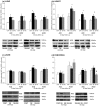
Renin angiotensin system (RAS) is known to play a key role in several diseases such as diabetes, and renal and cardiovascular pathologies. Its blockade has been demonstrated to delay chronic kidney disease progression and cardiovascular damage in diabetic patients. In this sense, since local RAS has been described, the aim of this study is to characterize angiotensin converting enzyme (ACE) and ACE2 activities, as well as protein expression, in several tissues of the non-obese diabetic (NOD) mice model. After 21 or 40 days of diabetes onset, mouse serums and tissues were analyzed for ACE and ACE2 enzyme activities and protein expression. ACE and ACE2 enzyme activities were detected in different tissues. Their expressions vary depending on the studied tissue. Thus, whereas ACE activity was highly expressed in lungs, ACE2 activity was highly expressed in pancreas among the studied tissues. Interestingly, we also observed that diabetes up-regulates ACE mainly in serum, lung, heart, and liver, and ACE2 mainly in serum, liver, and pancreas. In conclusion, we found a marked serum and pulmonary alteration in ACE activity of diabetic mice, suggesting a common regulation. The increase of ACE2 activity within the circulation in diabetic mice may be ascribed to a compensatory mechanism of RAS.
Keywords: angiotensin converting enzyme 2; diabetes; renin angiotensin system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87:E1–E9. doi: 10.1161/01.RES.87.5.e1. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

