Hybrid inorganic-organic capsules for efficient intracellular delivery of novel siRNAs against influenza A (H1N1) virus infection
- PMID: 28273907
- PMCID: PMC5427965
- DOI: 10.1038/s41598-017-00200-0
Hybrid inorganic-organic capsules for efficient intracellular delivery of novel siRNAs against influenza A (H1N1) virus infection
Abstract
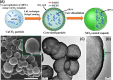
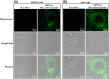
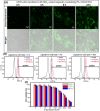
The implementation of RNAi technology into the clinical practice has been significantly postponing due to the issues regarding to the delivery of naked siRNA predominantly to target cells. Here we report the approach to enhance the efficiency of siRNA delivery by encapsulating the siRNA into new carrier systems which are obtained via the combination of widely used layer-by-layer technique and in situ modification by sol-gel chemistry. We used three types of siRNAs (NP-717, NP-1155 and NP-1496) in encapsulated form as new therapeutic agents against H1N1 influenza virus infection. By employing the hybrid microcontainers for the siRNA encapsulation we demonstrate the reduction of viral nucleoprotein (NP) level and inhibition of influenza virus production in infected cell lines (MDCK and A549). The obtained hybrid carriers based on assembled biodegradable polyelectrolytes and sol-gel coating possess several advantages such as a high cell uptake efficiency, low toxicity, efficient intracellular delivery of siRNAs and the protection of siRNAs from premature degradation before reaching the target cells. These findings underpin a great potential of versatile microencapsulation technology for the development of anti-viral RNAi delivery systems against influenza virus infection.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

