ALS-linked FUS exerts a gain of toxic function involving aberrant p38 MAPK activation
- PMID: 28273913
- PMCID: PMC5428330
- DOI: 10.1038/s41598-017-00091-1
ALS-linked FUS exerts a gain of toxic function involving aberrant p38 MAPK activation
Abstract
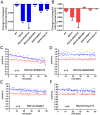
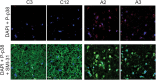
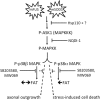
Mutations in Fused in Sarcoma/Translocated in Liposarcoma (FUS) cause familial forms of amyotrophic lateral sclerosis (ALS), a neurodegenerative disease characterized by progressive axonal degeneration mainly affecting motor neurons. Evidence from transgenic mouse models suggests mutant forms of FUS exert an unknown gain-of-toxic function in motor neurons, but mechanisms underlying this effect remain unknown. Towards this end, we studied the effect of wild type FUS (FUS WT) and three ALS-linked variants (G230C, R521G and R495X) on fast axonal transport (FAT), a cellular process critical for appropriate maintenance of axonal connectivity. All ALS-FUS variants impaired anterograde and retrograde FAT in squid axoplasm, whereas FUS WT had no effect. Misfolding of mutant FUS is implicated in this process, as the molecular chaperone Hsp110 mitigated these toxic effects. Interestingly, mutant FUS-induced impairment of FAT in squid axoplasm and of axonal outgrowth in mammalian primary motor neurons involved aberrant activation of the p38 MAPK pathway, as also reported for ALS-linked forms of Cu, Zn superoxide dismutase (SOD1). Accordingly, increased levels of active p38 MAPK were detected in post-mortem human ALS-FUS brain tissues. These data provide evidence for a novel gain-of-toxic function for ALS-linked FUS involving p38 MAPK activation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

