hERG S4-S5 linker acts as a voltage-dependent ligand that binds to the activation gate and locks it in a closed state
- PMID: 28273916
- PMCID: PMC5427910
- DOI: 10.1038/s41598-017-00155-2
hERG S4-S5 linker acts as a voltage-dependent ligand that binds to the activation gate and locks it in a closed state
Abstract
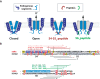
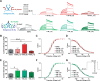
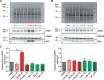
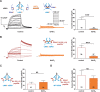
Delayed-rectifier potassium channels (hERG and KCNQ1) play a major role in cardiac repolarization. These channels are formed by a tetrameric pore (S5-S6) surrounded by four voltage sensor domains (S1-S4). Coupling between voltage sensor domains and the pore activation gate is critical for channel voltage-dependence. However, molecular mechanisms remain elusive. Herein, we demonstrate that covalently binding, through a disulfide bridge, a peptide mimicking the S4-S5 linker (S4-S5L) to the channel S6 C-terminus (S6T) completely inhibits hERG. This shows that channel S4-S5L is sufficient to stabilize the pore activation gate in its closed state. Conversely, covalently binding a peptide mimicking S6T to the channel S4-S5L prevents its inhibiting effect and renders the channel almost completely voltage-independent. This shows that the channel S4-S5L is necessary to stabilize the activation gate in its closed state. Altogether, our results provide chemical evidence that S4-S5L acts as a voltage-controlled ligand that binds S6T to lock the channel in a closed state, elucidating the coupling between voltage sensors and the gate in delayed rectifier potassium channels and potentially other voltage-gated channels.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

