Retinoic acid signaling is dispensable for somatic development and function in the mammalian ovary
- PMID: 28274610
- PMCID: PMC5411265
- DOI: 10.1016/j.ydbio.2017.02.015
Retinoic acid signaling is dispensable for somatic development and function in the mammalian ovary
Abstract
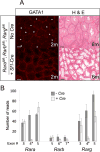
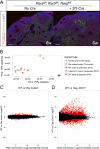
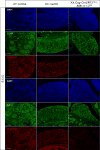
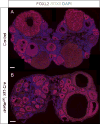
Retinoic acid (RA) is a potent inducer of cell differentiation and plays an essential role in sex-specific germ cell development in the mammalian gonad. RA is essential for male gametogenesis and hence fertility. However, RA can also disrupt sexual cell fate in somatic cells of the testis, promoting transdifferentiation of male Sertoli cells to female granulosa-like cells when the male sexual regulator Dmrt1 is absent. The feminizing ability of RA in the Dmrt1 mutant somatic testis suggests that RA might normally play a role in somatic cell differentiation or cell fate maintenance in the ovary. To test for this possibility we disrupted RA signaling in somatic cells of the early fetal ovary using three genetic strategies and one pharmaceutical approach. We found that deleting all three RA receptors (RARs) in the XX somatic gonad at the time of sex determination did not significantly affect ovarian differentiation, follicle development, or female fertility. Transcriptome analysis of adult triple mutant ovaries revealed remarkably little effect on gene expression in the absence of somatic RAR function. Likewise, deletion of three RA synthesis enzymes (Aldh1a1-3) at the time of sex determination did not masculinize the ovary. A dominant-negative RAR transgene altered granulosa cell proliferation, likely due to interference with a non-RA signaling pathway, but did not prevent granulosa cell specification and oogenesis or abolish fertility. Finally, culture of fetal XX gonads with an RAR antagonist blocked germ cell meiotic initiation but did not disrupt sex-biased gene expression. We conclude that RA signaling, although crucial in the ovary for meiotic initiation, is not required for granulosa cell specification, differentiation, or reproductive function.
Keywords: DMRT1; Granulosa; Ovary; Retinoic acid; Sertoli.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Ex vivo culture of human fetal gonads: manipulation of meiosis signalling by retinoic acid treatment disrupts testis development.Hum Reprod. 2015 Oct;30(10):2351-63. doi: 10.1093/humrep/dev194. Epub 2015 Aug 6. Hum Reprod. 2015. PMID: 26251460 Free PMC article.
-
Retinoic Acid signalling and the control of meiotic entry in the human fetal gonad.PLoS One. 2011;6(6):e20249. doi: 10.1371/journal.pone.0020249. Epub 2011 Jun 3. PLoS One. 2011. PMID: 21674038 Free PMC article.
-
Retinoic acid derived from the fetal ovary initiates meiosis in mouse germ cells.J Cell Physiol. 2013 Mar;228(3):627-39. doi: 10.1002/jcp.24172. J Cell Physiol. 2013. PMID: 22886539
-
Meiosis and retinoic acid in the mouse fetal gonads: An unforeseen twist.Curr Top Dev Biol. 2025;161:59-88. doi: 10.1016/bs.ctdb.2024.10.006. Epub 2024 Oct 29. Curr Top Dev Biol. 2025. PMID: 39870439 Review.
-
From Sex Determination to Initial Folliculogenesis in Mammalian Ovaries: Morphogenetic Waves along the Anteroposterior and Dorsoventral Axes.Sex Dev. 2015;9(4):190-204. doi: 10.1159/000440689. Epub 2015 Sep 25. Sex Dev. 2015. PMID: 26401661 Review.
Cited by
-
Morphometric analyses and gene expression related to germ cells, gonadal ridge epithelial-like cells and granulosa cells during development of the bovine fetal ovary.PLoS One. 2019 Mar 22;14(3):e0214130. doi: 10.1371/journal.pone.0214130. eCollection 2019. PLoS One. 2019. PMID: 30901367 Free PMC article.
-
Retinoic acid signaling in mouse retina endothelial cells is required for early angiogenic growth.Differentiation. 2023 Mar-Apr;130:16-27. doi: 10.1016/j.diff.2022.12.002. Epub 2022 Dec 14. Differentiation. 2023. PMID: 36528974 Free PMC article.
-
The pathogenic role of retinoid nuclear receptor signaling in cancer and metabolic syndromes.J Exp Med. 2024 Sep 2;221(9):e20240519. doi: 10.1084/jem.20240519. Epub 2024 Aug 12. J Exp Med. 2024. PMID: 39133222 Free PMC article. Review.
-
AOP Key Event Relationship report: Linking decreased retinoic acid levels with disrupted meiosis in developing oocytes.Curr Res Toxicol. 2022 Mar 18;3:100069. doi: 10.1016/j.crtox.2022.100069. eCollection 2022. Curr Res Toxicol. 2022. PMID: 35345548 Free PMC article.
-
Dissecting Human Gonadal Cell Lineage Specification and Sex Determination Using A Single-cell RNA-seq Approach.Genomics Proteomics Bioinformatics. 2022 Apr;20(2):223-245. doi: 10.1016/j.gpb.2022.04.002. Epub 2022 May 2. Genomics Proteomics Bioinformatics. 2022. PMID: 35513251 Free PMC article.
References
-
- Andersen B, Rosenfeld MG. Intracellular receptors. New wrinkles in retinoids. Nature. 1995;374:118–119. - PubMed
-
- Bagavandoss P, Midgley AR., Jr Biphasic action of retinoids on gonadotropin receptor induction in rat granulosa cells in vitro. Life Sci. 1988;43:1607–1614. - PubMed
-
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. - PubMed
-
- Bouma GJ, Affourtit JP, Bult CJ, Eicher EM. Transcriptional profile of mouse pre-granulosa and Sertoli cells isolated from early-differentiated fetal gonads. Gene Expr Patterns. 2007;7:113–123. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

