Early-life inflammation, immune response and ageing
- PMID: 28275145
- PMCID: PMC5360934
- DOI: 10.1098/rspb.2017.0125
Early-life inflammation, immune response and ageing
Erratum in
-
Correction to 'Early life inflammation, immune response and ageing'.Proc Biol Sci. 2017 Apr 12;284(1852):20170574. doi: 10.1098/rspb.2017.0574. Proc Biol Sci. 2017. PMID: 28404784 Free PMC article. No abstract available.
Abstract
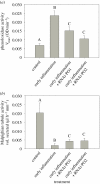
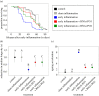
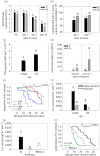
Age-related diseases are often attributed to immunopathology, which results in self-damage caused by an inappropriate inflammatory response. Immunopathology associated with early-life inflammation also appears to cause faster ageing, although we lack direct experimental evidence for this association. To understand the interactions between ageing, inflammation and immunopathology, we used the mealworm beetle Tenebrio molitor as a study organism. We hypothesized that phenoloxidase, an important immune effector in insect defence, may impose substantial immunopathological costs by causing tissue damage to Malpighian tubules (MTs; functionally equivalent to the human kidney), in turn accelerating ageing. In support of this hypothesis, we found that RNAi knockdown of phenoloxidase (PO) transcripts in young adults possibly reduced inflammation-induced autoreactive tissue damage to MTs, and increased adult lifespan. Our work thus suggests a causative link between immunopathological costs of early-life inflammation and faster ageing. We also reasoned that if natural selection weakens with age, older individuals should display increased immunopathological costs associated with an immune response. Indeed, we found that while old infected individuals cleared infection faster than young individuals, possibly they also displayed exacerbated immunopathological costs (larger decline in MT function) and higher post-infection mortality. RNAi-mediated knockdown of PO response partially rescued MTs function in older beetles and resulted in increased lifespan after infection. Taken together, our data are consistent with a direct role of immunopathological consequences of immune response during ageing in insects. Our work is also the first report that highlights the pervasive role of tissue damage under diverse contexts of ageing and immune response.
Keywords: Malpighian tubules; ageing; immunopathology; infection; inflammation.
© 2017 The Author(s).
Figures
References
-
- Nappi AJ, Vass E, Frey F, Carton Y. 1995. Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur. J. Cell Biol. 68, 450–456. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

