HCV RNA Testing of Plasma Samples from Cornea Donors: Suitability of Plasma Samples Stored at 4 °C for up to 8 Days
- PMID: 28275332
- PMCID: PMC5318926
- DOI: 10.1159/000449207
HCV RNA Testing of Plasma Samples from Cornea Donors: Suitability of Plasma Samples Stored at 4 °C for up to 8 Days
Abstract
Background: The HCV RNA testing of potential cornea donors frequently relies on blood samples stored pre mortem. The recommended storage time of maximum 72 h frequently excludes a significant fraction of donors.
Methods: The influence of storage time of EDTA plasma samples at 4 °C on the viral load measured with the Roche HCV Quantitative Test vs. 2.0 was evaluated for 43 samples from HCV-positive individuals.
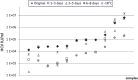
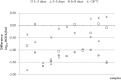
Results: The mean reduction of the viral load after 4 °C storage for 6-8 days was 0.46 log10 IU/ml (range +0.17 to -1.66 log10 IU/ml). After 1-3 days a mean loss of 0.19 log10 IU/ml (range +0.30 to -1.41 log10 IU/ml) and after 3-5 days of 0.32 log10 IU/ml (range +0.36 to -1.81 log10 IU/ml) was observed. In 23.3% of samples, a viral load reduction ≥ 1 log10 IU/ml (1.0-1.81 log10 IU/ml) was found after prolonged storage (5-8 days). In none of the samples did the HCV load fall below the detection limit.
Conclusion: Plasma storage for up to 8 days can quantitatively reduce the HCV RNA load, yet has no influence on the reliability of a qualitative HCV RNA detection by this ultrasensitive test to determine the HCV status of serologically negative cornea donors.
Keywords: Cornea donor testing; HCV-PCR; Prolonged sample storage at 4 °C.
Figures
References
-
- European Union Directive 2004/23/EC of the European Parliament and of the Council of 31 March 2004 on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells. Official Journal of the European Union. 2004;102:48–58.
-
- European Union Commission Directive 2006/17/EC of 8 February 2006 implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells. Official Journal of the European Union. 2006;38:40–52.
-
- Verordnung über die Anforderungen an Qualität und Sicherheit der Entnahme von Geweben und deren Übertragung nach dem Transplantationsgesetz (TPG-Gewebeverordnung – TPG-GewV). Fassung vom 26. März 2008;BGBl. I 1998;512.
-
- Paul-Ehrlich-Institut Beschluss der Bundesärztekammer vom 14.02.2014: Richtlinie zur Gewinnung von Spenderhornhäuten und zum Führen einer Augenhornhautbank. Dtsch Arztebl. 2014 DOI: 10.3238/arztebl.2014.rl_augenhornhautbank_01.
-
- Heim A, Wagner D, Rothämel T, Hartmann U, Flik J, Verhagen W. Evaluation of serological screening of cadaveric sera for donor selection for cornea transplantation. J Med Virol. 1999;58:291–295. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

