Advances in the Evaluation of Respiratory Pathophysiology during Exercise in Chronic Lung Diseases
- PMID: 28275353
- PMCID: PMC5319975
- DOI: 10.3389/fphys.2017.00082
Advances in the Evaluation of Respiratory Pathophysiology during Exercise in Chronic Lung Diseases
Abstract
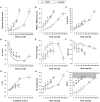
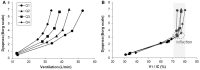
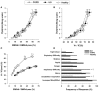
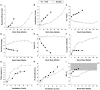
Dyspnea and exercise limitation are among the most common symptoms experienced by patients with various chronic lung diseases and are linked to poor quality of life. Our understanding of the source and nature of perceived respiratory discomfort and exercise intolerance in chronic lung diseases has increased substantially in recent years. These new mechanistic insights are the primary focus of the current review. Cardiopulmonary exercise testing (CPET) provides a unique opportunity to objectively evaluate the ability of the respiratory system to respond to imposed incremental physiological stress. In addition to measuring aerobic capacity and quantifying an individual's cardiac and ventilatory reserves, we have expanded the role of CPET to include evaluation of symptom intensity, together with a simple "non-invasive" assessment of relevant ventilatory control parameters and dynamic respiratory mechanics during standardized incremental tests to tolerance. This review explores the application of the new advances in the clinical evaluation of the pathophysiology of exercise intolerance in chronic obstructive pulmonary disease (COPD), chronic asthma, interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH). We hope to demonstrate how this novel approach to CPET interpretation, which includes a quantification of activity-related dyspnea and evaluation of its underlying mechanisms, enhances our ability to meaningfully intervene to improve quality of life in these pathologically-distinct conditions.
Keywords: chronic obstructive pulmonary disease; dyspnea; exercise; interstitial lung disease; pulmonary mechanics; pulmonary vascular diseases.
Figures





References
-
- Aaron E. A., Seow K. C., Johnson B. D., Dempsey J. A. (1992). Oxygen cost of exercise hyperpnea: implications for performance. J. Appl. Physiol. 72, 1818–1825. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

