A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging
- PMID: 28275562
- PMCID: PMC5337187
- DOI: 10.21037/qims.2017.02.09
A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging
Erratum in
-
Erratum to a comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging.Quant Imaging Med Surg. 2017 Jun;7(3):383. doi: 10.21037/qims.2017.05.05. Quant Imaging Med Surg. 2017. PMID: 28812007 Free PMC article.
Abstract
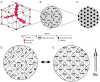
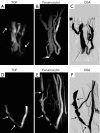
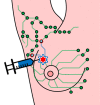
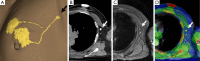
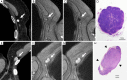
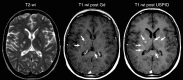
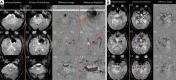
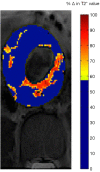
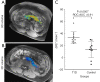
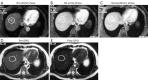
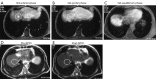
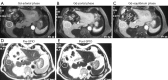
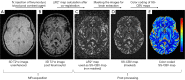
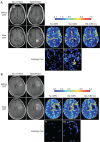
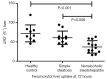
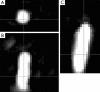
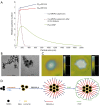
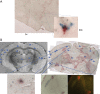
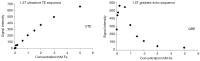
This paper aims to update the clinical researches using superparamagnetic iron oxide (SPIO) nanoparticles as magnetic resonance imaging (MRI) contrast agent published during the past five years. PubMed database was used for literature search, and the search terms were (SPIO OR superparamagnetic iron oxide OR Resovist OR Ferumoxytol OR Ferumoxtran-10) AND (MRI OR magnetic resonance imaging). The literature search results show clinical research on SPIO remains robust, particularly fuelled by the approval of ferumoxytol for intravenously administration. SPIOs have been tested on MR angiography, sentinel lymph node detection, lymph node metastasis evaluation; inflammation evaluation; blood volume measurement; as well as liver imaging. Two experimental SPIOs with unique potentials are also discussed in this review. A curcumin-conjugated SPIO can penetrate brain blood barrier (BBB) and bind to amyloid plaques in Alzheime's disease transgenic mice brain, and thereafter detectable by MRI. Another SPIO was fabricated with a core of Fe3O4 nanoparticle and a shell coating of concentrated hydrophilic polymer brushes and are almost not taken by peripheral macrophages as well as by mononuclear phagocytes and reticuloendothelial system (RES) due to the suppression of non-specific protein binding caused by their stealthy ''brush-afforded'' structure. This SPIO may offer potentials for the applications such as drug targeting and tissue or organ imaging other than liver and lymph nodes.
Keywords: MR angiography, sentinel lymph node; Superparamagnetic resonance iron oxide; contrast agent; lymph node metastasis; magnetic resonance imaging (MRI); superparamagnetic iron oxide (SPIO).
Conflict of interest statement
Conflicts of Interest: JM Idée is an employee of Guerbet group, France. Guerbet group manufactures and markets a number of contrast agents for diagnostic and interventional imaging. And other author has no conflicts of interest to declare.
Figures




References
-
- Wang YX. Chapter 2: Superparamagnetic iron oxide particle nanoparticles for cellular imaging and targeted therapy: Opportunities and changes for clinical translation. In: Zhang B. editor. Nano Imaging: From fundamental Principles to Translational Medical Applications. Singapore: World Scientific, 2017:29-51.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
