Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder
- PMID: 28275689
- PMCID: PMC5331780
- DOI: 10.1016/j.jcmgh.2016.11.008
Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder
Abstract
Background & aims: Emerging data on the gut microbiome in autism spectrum disorder (ASD) suggest that altered host-microbe interactions may contribute to disease symptoms. Although gut microbial communities in children with ASD are reported to differ from individuals with neurotypical development, it is not known whether these bacteria induce pathogenic neuroimmune signals.
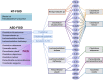
Methods: Because commensal clostridia interactions with the intestinal mucosa can regulate disease-associated cytokine and serotonergic pathways in animal models, we evaluated whether microbiome-neuroimmune profiles (from rectal biopsy specimens and blood) differed in ASD children with functional gastrointestinal disorders (ASD-FGID, n = 14) compared with neurotypical (NT) children with FGID (NT-FGID, n = 15) and without abdominal pain (NT, n = 6). Microbial 16S ribosomal DNA community signatures, cytokines, and serotonergic metabolites were quantified and correlated with gastrointestinal symptoms.
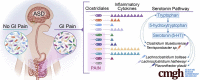
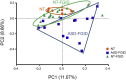
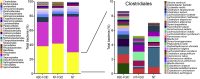
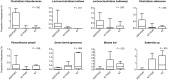
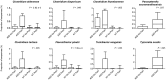
Results: A significant increase in several mucosa-associated Clostridiales was observed in ASD-FGID, whereas marked decreases in Dorea and Blautia, as well as Sutterella, were evident. Stratification by abdominal pain showed multiple organisms in ASD-FGID that correlated significantly with cytokines (interleukin [IL]6, IL1, IL17A, and interferon-γ). Group comparisons showed that IL6 and tryptophan release by mucosal biopsy specimens was highest in ASD children with abdominal pain, whereas serotonergic metabolites generally were increased in children with FGIDs. Furthermore, proinflammatory cytokines correlated significantly with several Clostridiales previously reported to associate with ASD, as did tryptophan and serotonin.
Conclusions: Our findings identify distinctive mucosal microbial signatures in ASD children with FGID that correlate with cytokine and tryptophan homeostasis. Future studies are needed to establish whether these disease-associated Clostridiales species confer early pathogenic signals in children with ASD and FGID.
Keywords: 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, serotonin; ASD, autism spectrum disorder; FGID, functional gastrointestinal disorder; GI, gastrointestinal; GM-CSF, granulocyte-macrophage colony-stimulating factor; GROα, growth-related oncogene alpha; Gastrointestinal Disorders; IBS, irritable bowel syndrome; IFN, interferon; IL, interleukin; IP, interferon gamma-induced protein; MCP-1, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; Microbiome; Microbiome–Gut–Brain Axis; Mucosa; NT, neurotypical; OTU, operational taxonomic unit; QPGS-RIII, Questionnaire on Pediatric Gastrointestinal Symptoms-Rome III; Serotonin; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Figures
References
-
- Buie T., Campbell D.B., Fuchs G.J., 3rd Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125(Suppl 1):S1–S18. - PubMed
-
- McElhanon B.O., McCracken C., Karpen S. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133:872–883. - PubMed
-
- Horvath K., Papadimitriou J.C., Rabsztyn A. Gastrointestinal abnormalities in children with autistic disorder. J Pediatr. 1999;135:559–563. - PubMed
-
- Fulceri F., Morelli M., Santocchi E. Gastrointestinal symptoms and behavioral problems in preschoolers with autism spectrum disorder. Dig Liver Dis. 2016;48:248–254. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

