Intrastriatally Infused Exogenous CDNF Is Endocytosed and Retrogradely Transported to Substantia Nigra
- PMID: 28275710
- PMCID: PMC5318544
- DOI: 10.1523/ENEURO.0128-16.2017
Intrastriatally Infused Exogenous CDNF Is Endocytosed and Retrogradely Transported to Substantia Nigra
Abstract
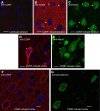
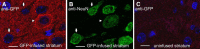
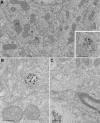
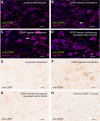
Cerebral dopamine neurotrophic factor (CDNF) protects the nigrostriatal dopaminergic (DA) neurons in rodent models of Parkinson's disease and restores DA circuitry when delivered after these neurons have begun to degenerate. These DA neurons have been suggested to transport striatal CDNF retrogradely to the substantia nigra (SN). However, in cultured cells the binding and internalization of extracellular CDNF has not been reported. The first aim of this study was to examine the cellular localization and pharmacokinetic properties of recombinant human CDNF (rhCDNF) protein after its infusion into rat brain parenchyma. Second, we aimed to study whether the transport of rhCDNF from the striatum to the SN results from its retrograde transport via DA neurons or from its anterograde transport via striatal GABAergic projection neurons. We show that after intrastriatal infusion, rhCDNF diffuses rapidly and broadly, and is cleared with a half-life of 5.5 h. Confocal microscopy analysis of brain sections at 2 and 6 h after infusion of rhCDNF revealed its widespread unspecific internalization by cortical and striatal neurons, exhibiting different patterns of subcellular rhCDNF distribution. Electron microscopy analysis showed that rhCDNF is present inside the endosomes and multivesicular bodies. In addition, we present data that after intrastriatal infusion the rhCDNF found in the SN is almost exclusively localized to the DA neurons, thus showing that it is retrogradely transported.
Keywords: CDNF; Parkinson’s disease.
Figures



Similar articles
-
Novel neurotrophic factor CDNF protects and rescues midbrain dopamine neurons in vivo.Nature. 2007 Jul 5;448(7149):73-7. doi: 10.1038/nature05957. Nature. 2007. PMID: 17611540
-
Evidence for an Additive Neurorestorative Effect of Simultaneously Administered CDNF and GDNF in Hemiparkinsonian Rats: Implications for Different Mechanism of Action.eNeuro. 2017 Mar 13;4(1):ENEURO.0117-16.2017. doi: 10.1523/ENEURO.0117-16.2017. eCollection 2017 Jan-Feb. eNeuro. 2017. PMID: 28303260 Free PMC article.
-
Enhanced neurotrophic distribution, cell signaling and neuroprotection following substantia nigral versus striatal delivery of AAV2-NRTN (CERE-120).Neurobiol Dis. 2013 Oct;58:38-48. doi: 10.1016/j.nbd.2013.04.011. Epub 2013 Apr 28. Neurobiol Dis. 2013. PMID: 23631873
-
Unconventional neurotrophic factors CDNF and MANF: Structure, physiological functions and therapeutic potential.Neurobiol Dis. 2017 Jan;97(Pt B):90-102. doi: 10.1016/j.nbd.2016.07.009. Epub 2016 Jul 15. Neurobiol Dis. 2017. PMID: 27425895 Review.
-
CDNF and ER stress: Pharmacology and therapeutic possibilities.Pharmacol Ther. 2024 Feb;254:108594. doi: 10.1016/j.pharmthera.2024.108594. Epub 2024 Jan 28. Pharmacol Ther. 2024. PMID: 38290651 Review.
Cited by
-
Beneficial behavioral effects of chronic cerebral dopamine neurotrophic factor (CDNF) infusion in the N171-82Q transgenic model of Huntington's disease.Sci Rep. 2023 Feb 20;13(1):2953. doi: 10.1038/s41598-023-28798-4. Sci Rep. 2023. PMID: 36807563 Free PMC article.
-
Key Subdomains of Cerebral Dopamine Neurotrophic Factor Regulate Its Protective Function in 6-Hydroxydopamine-Lesioned PC12 Cells.DNA Cell Biol. 2023 Nov;42(11):680-688. doi: 10.1089/dna.2023.0215. Epub 2023 Oct 9. DNA Cell Biol. 2023. PMID: 37815547 Free PMC article.
-
Cerebral dopamine neurotrophic factor (CDNF) protects against quinolinic acid-induced toxicity in in vitro and in vivo models of Huntington's disease.Sci Rep. 2020 Nov 5;10(1):19045. doi: 10.1038/s41598-020-75439-1. Sci Rep. 2020. PMID: 33154393 Free PMC article.
-
Hormonal therapies up-regulate MANF and overcome female susceptibility to immune checkpoint inhibitor myocarditis.Sci Transl Med. 2022 Nov 2;14(669):eabo1981. doi: 10.1126/scitranslmed.abo1981. Epub 2022 Nov 2. Sci Transl Med. 2022. PMID: 36322628 Free PMC article.
-
Navigating the Landscape of MANF Research: A Scientometric Journey with CiteSpace Analysis.Cell Mol Neurobiol. 2023 Nov;43(8):3897-3913. doi: 10.1007/s10571-023-01412-x. Epub 2023 Sep 26. Cell Mol Neurobiol. 2023. PMID: 37751132 Free PMC article. Review.
References
-
- Airavaara M, Harvey BK, Voutilainen MH, Shen H, Chou J, Lindholm P, Lindahl M, Tuominen RK, Saarma M, Hoffer B, Wang Y (2012) CDNF protects the nigrostriatal dopamine system and promotes recovery after MPTP treatment in mice. Cell Transplant 21:1213–1223. 10.3727/096368911X600948 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
