Comparative Effectiveness of Adding Alogliptin to Metformin Plus Sulfonylurea with Other DPP-4 Inhibitors in Type 2 Diabetes: A Systematic Review and Network Meta-Analysis
- PMID: 28275958
- PMCID: PMC5380505
- DOI: 10.1007/s13300-017-0245-8
Comparative Effectiveness of Adding Alogliptin to Metformin Plus Sulfonylurea with Other DPP-4 Inhibitors in Type 2 Diabetes: A Systematic Review and Network Meta-Analysis
Abstract
Introduction: Alogliptin is an oral antihyperglycemic agent that is a selective inhibitor of the enzyme dipeptidyl peptidase-4 (DPP-4), approved for the treatment of type 2 diabetes mellitus (T2DM). There currently exists no comparative data to support the use of alogliptin in combination with metformin (met) and sulfonylurea (SU). A decision-focused network meta-analysis (NMA) was performed to compare the relative efficacy and safety of alogliptin 25 mg once daily to other DPP-4 inhibitors as part of a triple therapy regimen for patients inadequately controlled on metformin and SU dual therapy.
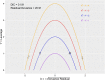
Methods: A systematic literature review was conducted to identify published papers of randomized controlled trials (RCTs) that compared alogliptin with other DPP-4 inhibitors (linagliptin, saxagliptin, sitagliptin, and vildagliptin) at their Summary of Product Characteristics (SmPC) recommended daily doses, added on to metformin and SU. Comprehensive comparative analysis involving frequentist meta-analysis and Bayesian NMA compared alogliptin to each DPP-4 inhibitor separately and collectively as a group. Quasi-random effect models were introduced when random effect models could not be estimated.
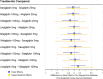
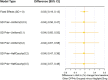
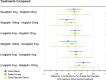
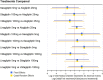
Results: The review identified 2186 articles, and 94 full-text articles were assessed for eligibility. Eight RCTs contained appropriate data for inclusion in the NMA. All analyses over all trial population sets produced very similar results, and show that alogliptin 25 mg is as least as effective (as measured by change in HbA1c from baseline, but supported by other outcome measures: change in body weight and FPG from baseline) and safe (as measured by incidence of hypoglycemia and adverse events leading to study discontinuation) as all the other DPP-4 inhibitors in triple therapy.
Conclusion: This decision-focused systematic review and NMA demonstrated alogliptin 25 mg daily to have similar efficacy and safety compared to other DPP-4 inhibitors, for the treatment of T2DM in adults inadequately controlled on metformin and SU. (Funded by Takeda Development Centre Americas; EXAMINE ClinicalTrials.gov number, NCT00968708).
Keywords: Alogliptin once daily; DPP-4 inhibitor; Network meta-analysis; Systematic review; Triple therapy; Type 2 diabetes mellitus.
Figures







References
-
- European Medicines Agency. CHMP assessment report. Vipidia. EMA/CHMP/207780/2013. 25 July 2013.
-
- Nauck MA, Ellis GC, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of adding the dipeptidyl peptidase-4 inhibitor alogliptin to metformin therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a multicentre, randomised, double-blind, placebo-controlled study. Int J Clin Pract. 2009;63(1):46–55. doi: 10.1111/j.1742-1241.2008.01933.x. - DOI - PubMed
-
- Pratley RE, Reusch JE, Fleck PR, Wilson CA, Mekki Q. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor alogliptin added to pioglitazone in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2009;25(10):2361–2371. doi: 10.1185/03007990903156111. - DOI - PubMed
-
- Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9(5):733–745. doi: 10.1111/j.1463-1326.2007.00744.x. - DOI - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

