The Shc1 adaptor simultaneously balances Stat1 and Stat3 activity to promote breast cancer immune suppression
- PMID: 28276425
- PMCID: PMC5347092
- DOI: 10.1038/ncomms14638
The Shc1 adaptor simultaneously balances Stat1 and Stat3 activity to promote breast cancer immune suppression
Abstract
Tyrosine kinase signalling within cancer cells is central to the establishment of an immunosuppressive microenvironment. Although tyrosine kinase inhibitors act, in part, to augment adaptive immunity, the increased heterogeneity and functional redundancy of the tyrosine kinome is a hurdle to achieving durable responses to immunotherapies. We previously identified the Shc1 (ShcA) scaffold, a central regulator of tyrosine kinase signalling, as essential for promoting breast cancer immune suppression. Herein we show that the ShcA pathway simultaneously activates STAT3 immunosuppressive signals and impairs STAT1-driven immune surveillance in breast cancer cells. Impaired Y239/Y240-ShcA phosphorylation selectively reduces STAT3 activation in breast tumours, profoundly sensitizing them to immune checkpoint inhibitors and tumour vaccines. Finally, the ability of diminished tyrosine kinase signalling to initiate STAT1-driven immune surveillance can be overcome by compensatory STAT3 hyperactivation in breast tumours. Our data indicate that inhibition of pY239/240-ShcA-dependent STAT3 signalling may represent an attractive therapeutic strategy to sensitize breast tumours to multiple immunotherapies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



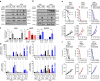
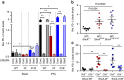


References
-
- Smyth M. J., Ngiow S. F., Ribas A. & Teng M. W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016). - PubMed
-
- Robert C. et al.. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011). - PubMed
-
- Finak G. et al.. Stromal gene expression predicts clinical outcome in breast cancer. Nat. Med. 14, 518–527 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

