Antimicrobial peptide cWFW kills by combining lipid phase separation with autolysis
- PMID: 28276520
- PMCID: PMC5343580
- DOI: 10.1038/srep44332
Antimicrobial peptide cWFW kills by combining lipid phase separation with autolysis
Abstract
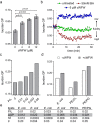
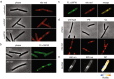
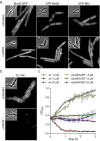
The synthetic cyclic hexapeptide cWFW (cyclo(RRRWFW)) has a rapid bactericidal activity against both Gram-positive and Gram-negative bacteria. Its detailed mode of action has, however, remained elusive. In contrast to most antimicrobial peptides, cWFW neither permeabilizes the membrane nor translocates to the cytoplasm. Using a combination of proteome analysis, fluorescence microscopy, and membrane analysis we show that cWFW instead triggers a rapid reduction of membrane fluidity both in live Bacillus subtilis cells and in model membranes. This immediate activity is accompanied by formation of distinct membrane domains which differ in local membrane fluidity, and which severely disrupts membrane protein organisation by segregating peripheral and integral proteins into domains of different rigidity. These major membrane disturbances cause specific inhibition of cell wall synthesis, and trigger autolysis. This novel antibacterial mode of action holds a low risk to induce bacterial resistance, and provides valuable information for the design of new synthetic antimicrobial peptides.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Baltzer S. A. & Brown M. H. Antimicrobial peptides: Promising alternatives to conventional antibiotics. J. Mol. Microbiol. Biotechnol. 20, 228–235 (2011). - PubMed
-
- Appelt C., Wessolowski A., Dathe M. & Schmieder P. Structures of cyclic, antimicrobial peptides in a membrane-mimicking environment define requirements for activity. J. Pept. Sci. 14, 524–527 (2008). - PubMed
-
- Junkes C. et al.. Cyclic antimicrobial R-, W-rich peptides: The role of peptide structure and E. coli outer and inner membranes in activity and the mode of action. Eur. Biophys. J. 40, 515–528 (2011). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

