Force degradation behavior of glucocorticoid deflazacort by UPLC: isolation, identification and characterization of degradant by FTIR, NMR and mass analysis
- PMID: 28276670
- PMCID: PMC4820892
- DOI: 10.7555/JBR.30.20150074
Force degradation behavior of glucocorticoid deflazacort by UPLC: isolation, identification and characterization of degradant by FTIR, NMR and mass analysis
Abstract
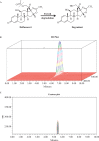
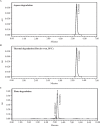
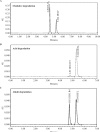
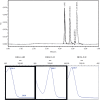
In this investigation, sensitive and reproducible methods are described for quantitative determination of deflazacort in the presence of its degradation product. The method was based on high performance liquid chromatography of the drug from its degradation product on reverse phase using Acquity UPLC BEH C18 columns (1.7 µm, 2.1 mm × 150 mm) using acetonitrile and water (40:60 V/V) at a flow rate of 0.2 mL/minute in UPLC. UV detection was performed at 240.1 nm. Deflazacort was subjected to oxidative, acid, base, hydrolytic, thermal and photolytic degradation. The drug was found to be stable in water and thermal stress, as well as under neutral stress conditions. However, forced-degradation study performed on deflazacort showed that the drug degraded under alkaline, acid and photolytic stress. The degradation products were well resolved from the main peak, which proved the stability-indicating power of the method. The developed method was validated as per ICH guidelines with respect to accuracy, linearity, limit of detection, limit of quantification, accuracy, precision and robustness, selectivity and specificity. Apart from the aforementioned, the results of the present study also emphasize the importance of isolation characterization and identification of degradant. Hence, an attempt was made to identify the degradants in deflazacort. One of the degradation products of deflazacort was isolated and identified by the FTIR, NMR and LC-MS study.
Keywords: Deflazacort; characterization; degradant; forced degradation.
© 2016 by the Journal of Biomedical Research. All rights reserved.
Conflict of interest statement
CLC number: R917, Document code: A
The authors reported no conflict of interest.
Figures
Similar articles
-
UPLC and LC-MS studies on degradation behavior of irinotecan hydrochloride and development of a validated stability-indicating ultra-performance liquid chromatographic method for determination of irinotecan hydrochloride and its impurities in pharmaceutical dosage forms.J Chromatogr Sci. 2012 Oct;50(9):810-9. doi: 10.1093/chromsci/bms075. Epub 2012 Jun 1. J Chromatogr Sci. 2012. PMID: 22661461
-
Development and validation of a novel stability-indicating HPLC method for the quantitative determination of eleven related substances in ezetimibe drug substance and drug product.Talanta. 2015 Jul 1;139:67-74. doi: 10.1016/j.talanta.2015.02.039. Epub 2015 Feb 27. Talanta. 2015. PMID: 25882410
-
Identification and Characterization of an Oxidative Degradation Product of Fexofenadine, Development and Validation of a Stability-Indicating RP-UPLC Method for the Estimation of Process Related Impurities and Degradation Products of Fexofenadine in Pharmaceutical Formulations.Sci Pharm. 2012 Apr-Jun;80(2):295-309. doi: 10.3797/scipharm.1111-07. Epub 2012 Jan 21. Sci Pharm. 2012. PMID: 22896817 Free PMC article.
-
A validated stability-indicating UPLC method for desloratadine and its impurities in pharmaceutical dosage forms.J Pharm Biomed Anal. 2010 Feb 5;51(3):736-42. doi: 10.1016/j.jpba.2009.09.016. Epub 2009 Sep 20. J Pharm Biomed Anal. 2010. PMID: 19815361
-
Development of forced degradation and stability indicating studies of drugs-A review.J Pharm Anal. 2014 Jun;4(3):159-165. doi: 10.1016/j.jpha.2013.09.003. Epub 2013 Sep 17. J Pharm Anal. 2014. PMID: 29403878 Free PMC article. Review.
Cited by
-
Comparative Stability of Two Anti-hyperpigmentation Agents: Kojic Acid as a Natural Metabolite and Its Di-Palmitate Ester, Under Oxidative Stress; Application to Pharmaceutical Formulation Design.Adv Pharm Bull. 2022 Mar;12(2):329-335. doi: 10.34172/apb.2022.031. Epub 2021 May 30. Adv Pharm Bull. 2022. PMID: 35620332 Free PMC article. Review.
References
-
- Saviola G, Abdi Ali L, Shams Eddin S, et al. Compared clinical efficacy and bone metabolic effects of low-dose deflazacort and methyl prednisolone in male inflammatory arthropathies: a 12-month open randomized pilot study[J]. Rheumatology (Oxford) 2007466): 994–8 - PubMed
-
- Del Rosso A, Cinelli M, Guiducci S, et al. Deflazacort modulates the fibrinolytic pattern and reduces uPA-dependent chemioinvasion and proliferation in rheumatoid arthritis synoviocytes[J]. Rheumatology (Oxford) 200544101255–62 - PubMed
-
- Markham A, Bryson HM.Deflazacort. A review of its pharmacological properties and therapeutic efficacy[J]. Drugs 1995502317–33 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

