Predictors of Low Uptake of Prenatal Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Immunization in Privately Insured Women in the United States
- PMID: 28277354
- PMCID: PMC5364044
- DOI: 10.1097/AOG.0000000000001927
Predictors of Low Uptake of Prenatal Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis Immunization in Privately Insured Women in the United States
Abstract
Objective: To examine the uptake of prenatal tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) immunization among pregnant women in the United States.
Methods: Using MarketScan data, we conducted a historical cohort study among pregnant women with employer-based commercial insurance in the United States who delivered between January 1, 2010, and December 31, 2014. We examined temporal trends of uptake, predictors of uptake, and timing of Tdap immunization.
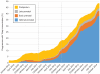
Results: Among 1,222,384 eligible pregnancies in 1,147,711 women, receipt of prenatal Tdap immunization increased from 0.0% of women who delivered in January 2010 to 9.8% who delivered in October 2012 (the date of the recommendation by the Advisory Committee on Immunization Practices for Tdap during every pregnancy) to 44.4% who delivered in December 2014. Among women who received Tdap during pregnancy, the majority were immunized between 27 weeks and 36 6/7 weeks of gestation per the Advisory Committee on Immunization Practices recommendation. In multivariable analyses among women who delivered between November 2012 and December 2014, rates of prenatal Tdap immunization were lower for women younger than 25 years of age (eg, 20-24 compared with 30-34 years rate ratio [RR] 0.83, 95% confidence interval [CI] 0.85-0.88), with other children (eg, three compared with zero children: RR 0.86, 95% CI 0.84-0.88), residing in the South compared with the Midwest (RR 0.81, 95% CI 0.80-0.82), or with emergency department visits in early pregnancy (RR 0.93, 95% CI 0.92-0.95). The proportion of pregnant women who received prenatal Tdap increased with increasing gestational age at birth.
Conclusion: By the end of 2014, fewer than half of pregnant women in the United States were receiving prenatal Tdap immunization. Implementation and dissemination strategies are needed to increase Tdap coverage among pregnant women, especially those who are young, have other children, or reside in the South.
Figures



Similar articles
-
Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel.MMWR Recomm Rep. 2006 Dec 15;55(RR-17):1-37. MMWR Recomm Rep. 2006. PMID: 17167397
-
Effectiveness of Prenatal Tetanus, Diphtheria, Acellular Pertussis Vaccination in the Prevention of Infant Pertussis in the U.S.Am J Prev Med. 2018 Aug;55(2):159-166. doi: 10.1016/j.amepre.2018.04.013. Epub 2018 Jun 14. Am J Prev Med. 2018. PMID: 29910115 Free PMC article.
-
Vaccination with tetanus, diphtheria, and acellular pertussis vaccine of pregnant women enrolled in Medicaid--Michigan, 2011-2013.MMWR Morb Mortal Wkly Rep. 2014 Sep 26;63(38):839-42. MMWR Morb Mortal Wkly Rep. 2014. PMID: 25254561 Free PMC article.
-
Prevention of pertussis, tetanus, and diphtheria among pregnant and postpartum women and their infants recommendations of the Advisory Committee on Immunization Practices (ACIP).MMWR Recomm Rep. 2008 May 30;57(RR-4):1-51. MMWR Recomm Rep. 2008. PMID: 18509304 Review.
-
Factors associated with Tdap vaccination receipt during pregnancy: a cross-sectional study.Public Health. 2020 Feb;179:38-44. doi: 10.1016/j.puhe.2019.10.001. Epub 2019 Nov 11. Public Health. 2020. PMID: 31726399 Review.
Cited by
-
Prenatal vaccination of mothers and hepatitis B vaccination of their infants.Prev Med. 2019 Apr;121:68-73. doi: 10.1016/j.ypmed.2019.02.013. Epub 2019 Feb 11. Prev Med. 2019. PMID: 30763628 Free PMC article.
-
Identifying pregnancies in insurance claims data: Methods and application to retinoid teratogenic surveillance.Pharmacoepidemiol Drug Saf. 2019 Sep;28(9):1211-1221. doi: 10.1002/pds.4794. Epub 2019 Jul 22. Pharmacoepidemiol Drug Saf. 2019. PMID: 31328328 Free PMC article.
-
Tetanus, Diphtheria, and Acellular Pertussis and Influenza Vaccinations among Women With a Live Birth, Internet Panel Survey, 2017-2018.Infect Dis (Auckl). 2020 Feb 10;13:1178633720904099. doi: 10.1177/1178633720904099. eCollection 2020. Infect Dis (Auckl). 2020. PMID: 32095076 Free PMC article.
-
Wildfire smoke exposure and early childhood respiratory health: a study of prescription claims data.Environ Health. 2023 Jun 27;22(1):48. doi: 10.1186/s12940-023-00998-5. Environ Health. 2023. PMID: 37370168 Free PMC article.
-
Factors associated with uptake of influenza and pertussis vaccines among pregnant women in South Australia.PLoS One. 2018 Jun 14;13(6):e0197867. doi: 10.1371/journal.pone.0197867. eCollection 2018. PLoS One. 2018. PMID: 29902184 Free PMC article.
References
-
- Centers for Disease Control and Prevention 2014 Provisional Pertussis Surveillance Report. 2015
-
- Cortese MM, Baughman AL, Zhang R, Srivastava PU, Wallace GS. Pertussis hospitalizations among infants in the United States, 1993 to 2004. Pediatrics. 2008;121(3):484–492. - PubMed
-
- Kline JM, Lewis WD, Smith EA, Tracy LR, Moerschel SK. Pertussis: a reemerging infection. American family physician. 2013;88(8):507–514. - PubMed
-
- Pertussis vaccination: use of acellular pertussis vaccines among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 1997;46(Rr-7):1–25. - PubMed
-
- Auerbach BS, Lake AM, Wilson ME, et al. Dose-response to a two-component acellular pertussis vaccine in infants and comparison with whole-cell vaccine. Biologicals : journal of the International Association of Biological Standardization. 1998;26(2):145–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

