p53 loss-of-heterozygosity is a necessary prerequisite for mutant p53 stabilization and gain-of-function in vivo
- PMID: 28277540
- PMCID: PMC5386572
- DOI: 10.1038/cddis.2017.80
p53 loss-of-heterozygosity is a necessary prerequisite for mutant p53 stabilization and gain-of-function in vivo
Abstract
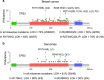
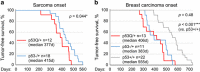
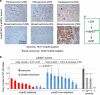
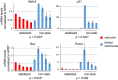
Missense mutations in TP53 comprise >75% of all p53 alterations in cancer, resulting in highly stabilized mutant p53 proteins that not only lose their tumor-suppressor activity, but often acquire oncogenic gain-of-functions (GOFs). GOF manifests itself in accelerated tumor onset, increased metastasis, increased drug resistance and shortened survival in patients and mice. A known prerequisite for GOF is mutant p53 protein stabilization, which itself is linked to aberrant protein conformation. However, additional determinants for mutant p53 stabilization likely exist. Here we show that in initially heterozygous mouse tumors carrying the hotspot GOF allele R248Q (p53Q/+), another necessary prerequisite for mutant p53 stabilization and GOF in vivo is loss of the remaining wild-type p53 allele, termed loss-of-heterozygosity (LOH). Thus, in mouse tumors with high frequency of p53 LOH (osteosarcomas and fibrosarcomas), we find that mutant p53 protein is stabilized (16/17 cases, 94%) and tumor onset is significantly accelerated compared with p53+/- tumors (GOF). In contrast, in mouse tumors with low frequency of p53 LOH (MMTV-Neu breast carcinomas), mutant p53 protein is not stabilized (16/20 cases, 80%) and GOF is not observed. Of note, human genomic databases (TCGA, METABRIC etc.) show a high degree of p53 LOH in all examined tumor types that carry missense p53 mutations, including sarcomas and breast carcinomas (with and without HER2 amplification). These data - while cautioning that not all genetic mouse models faithfully represent the human situation - demonstrate for the first time that p53 LOH is a critical prerequisite for missense mutant p53 stabilization and GOF in vivo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell 2004; 119: 861–872. - PubMed
-
- Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell 2004; 119: 847–860. - PubMed
-
- Doyle B, Morton JP, Delaney DW, Ridgway RA, Wilkins JA, Sansom OJ. p53 mutation and loss have different effects on tumourigenesis in a novel mouse model of pleomorphic rhabdomyosarcoma. J Pathol 2010; 222: 129–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

