Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals
- PMID: 28277543
- PMCID: PMC5386574
- DOI: 10.1038/cddis.2017.82
Estrogen receptors in granulosa cells govern meiotic resumption of pre-ovulatory oocytes in mammals
Abstract
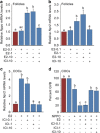
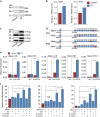
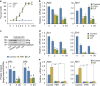
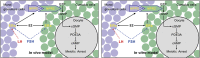
In mammals, oocytes are arrested at the diplotene stage of meiosis I until the pre-ovulatory luteinizing hormone (LH) surge triggers meiotic resumption through the signals in follicular granulosa cells. In this study, we show that the estradiol (E2)-estrogen receptors (ERs) system in follicular granulosa cells has a dominant role in controlling oocyte meiotic resumption in mammals. We found that the expression of ERs was controlled by gonadotropins under physiological conditions. E2-ERs system was functional in maintaining oocyte meiotic arrest by regulating the expression of natriuretic peptide C and natriuretic peptide receptor 2 (NPPC/NPR2), which was achieved through binding to the promoter regions of Nppc and Npr2 genes directly. In ER knockout mice, meiotic arrest was not sustained by E2 in most cumulus-oocyte complexes in vitro and meiosis resumed precociously in pre-ovulatory follicles in vivo. In human granulosa cells, similar conclusions are reached that ER levels were controlled by gonadotropins and E2-ERs regulated the expression of NPPC/NPR2 levels. In addition, our results revealed that the different regulating patterns of follicle-stimulating hormone and LH on ER levels in vivo versus in vitro determined their distinct actions on oocyte maturation. Taken together, these findings suggest a critical role of E2-ERs system during oocyte meiotic progression and may propose a novel approach for oocyte in vitro maturation treatment in clinical practice.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles.Mol Reprod Dev. 2012 Nov;79(11):795-802. doi: 10.1002/mrd.22114. Epub 2012 Sep 28. Mol Reprod Dev. 2012. PMID: 22987720
-
Pre-ovulatory LH/hCG surge decreases C-type natriuretic peptide secretion by ovarian granulosa cells to promote meiotic resumption of pre-ovulatory oocytes.Hum Reprod. 2011 Nov;26(11):3094-101. doi: 10.1093/humrep/der282. Epub 2011 Aug 23. Hum Reprod. 2011. PMID: 21865234
-
Transforming growth factor-β is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells.Cell Death Dis. 2019 Jul 22;10(8):558. doi: 10.1038/s41419-019-1797-5. Cell Death Dis. 2019. PMID: 31332164 Free PMC article.
-
Molecular control of oocyte meiotic arrest and resumption.Reprod Fertil Dev. 2013;25(3):463-71. doi: 10.1071/RD12310. Reprod Fertil Dev. 2013. PMID: 23217677 Review.
-
Nppc/Npr2/cGMP signaling cascade maintains oocyte developmental capacity.Cell Mol Biol (Noisy-le-grand). 2019 Apr 30;65(4):83-89. Cell Mol Biol (Noisy-le-grand). 2019. PMID: 31078160 Review.
Cited by
-
The role of Kisspeptin signaling in Oocyte maturation.Front Endocrinol (Lausanne). 2022 Aug 22;13:917464. doi: 10.3389/fendo.2022.917464. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36072937 Free PMC article. Review.
-
Luteinizing Hormone Action in Human Oocyte Maturation and Quality: Signaling Pathways, Regulation, and Clinical Impact.Reprod Sci. 2020 Jun;27(6):1223-1252. doi: 10.1007/s43032-019-00137-x. Epub 2020 Jan 6. Reprod Sci. 2020. PMID: 32046451 Free PMC article. Review.
-
Rescue in vitro maturation using ovarian support cells of human oocytes from conventional stimulation cycles yields oocytes with improved nuclear maturation and transcriptomic resemblance to in vivo matured oocytes.J Assist Reprod Genet. 2024 Aug;41(8):2021-2036. doi: 10.1007/s10815-024-03143-4. Epub 2024 May 30. J Assist Reprod Genet. 2024. PMID: 38814543 Free PMC article.
-
Molecular evidence for pre-chordate origins of ovarian cell types and neuroendocrine control of reproduction.bioRxiv [Preprint]. 2025 Mar 28:2025.03.24.644836. doi: 10.1101/2025.03.24.644836. bioRxiv. 2025. PMID: 40196654 Free PMC article. Preprint.
-
Comparative Transcriptome Profiling of mRNA and lncRNA of Ovaries in High and Low Egg Production Performance in Domestic Pigeons (Columba livia).Front Genet. 2021 Mar 23;12:571325. doi: 10.3389/fgene.2021.571325. eCollection 2021. Front Genet. 2021. PMID: 33833772 Free PMC article.
References
-
- Kiyosu C, Tsuji T, Yamada K, Kajita S, Kunieda T. NPPC/NPR2 signaling is essential for oocyte meiotic arrest and cumulus oophorus formation during follicular development in the mouse ovary. Reproduction 2012; 144: 187–193. - PubMed
-
- Sela-Abramovich S, Edry I, Galiani D, Nevo N, Dekel N. Disruption of gap junctional communication within the ovarian follicle induces oocyte maturation. Endocrinology 2006; 147: 2280–2286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

