miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase 1
- PMID: 28277545
- PMCID: PMC5386568
- DOI: 10.1038/cddis.2017.75
miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase 1
Erratum in
-
Correction: miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase 1.Cell Death Dis. 2019 Nov 12;10(11):859. doi: 10.1038/s41419-019-2097-9. Cell Death Dis. 2019. PMID: 31719519 Free PMC article.
-
Correction: miR-377 induces senescence in human skin fibroblasts by targeting DNA methyltransferase 1.Cell Death Dis. 2025 Nov 17;16(1):835. doi: 10.1038/s41419-025-08184-w. Cell Death Dis. 2025. PMID: 41249162 Free PMC article. No abstract available.
Abstract
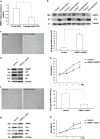
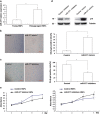
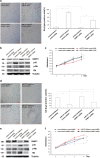
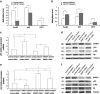
Skin aging is a complicated physiological process and epigenetic feature, including microRNA-mediated regulation and DNA methylation, have been shown to contribute to this process. DNA methylation is regulated by DNA methyltransferase, of which DNA methyltransferase 1 (DNMT1) is the most abundantly known. But evidence supporting its role in skin aging remains scarce, and no report regards its specifical upstream-regulating molecules in the process of skin aging so far. Here, we found that DNMT1 expression was markedly higher in young human skin fibroblasts (HSFs) than that in passage-aged HSFs, and DNMT1 knockdown significantly induced the senescence phenotype in young HSFs. We predicted the upstream miRNAs which could regulate DNMT1 with miRNA databases and found miR-377 had high homology with a sequence in the 3'-UTR of human DNMT1 mRNA. We confirmed that miR-377 was a potential regulator of DNMT1 by luciferase reporter assays. miR-377 expression in passage-aged HSFs was markedly higher than that in the young HSFs. miR-377 overexpression promoted senescence in young HSFs, and inhibition of miR-377 reduced senescence in passage-aged HSFs. Moreover, these functions were mediated by targeting DNMT1. Microfluidic PCR and next-generation bisulfite sequencing of 24 senescent-associated genes' promoters revealed alterations of the promoter methylation levels of FoxD3, p53, and UTF1 in HSFs treated with miR-377 mimics or inhibitors. We also verified that the miR-377-mediated changes in p53 expression could be reversed by regulation of DNMT1 in HSFs. Similarly, there was a negative correlation between miR-377 and DNMT1 expression in young and photoaged HSFs, HSFs, or skin tissues from UV-unexposed areas of different aged donors. Our results highlight a novel role for miR-377-DNMT1-p53 axis in HSF senescence. These findings shed new light on the mechanisms of skin aging and identify future opportunities for its therapeutic prevention.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

